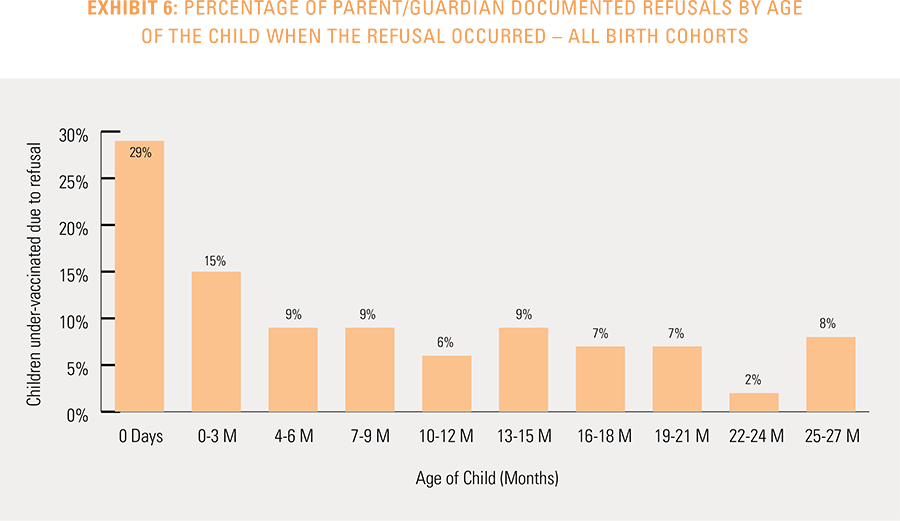Appendix B: Methodology
Data and Study Population
This is the seventeenth study of the Blue Cross Blue Shield: The Health of America Report series, a collaboration between the Blue Cross Blue Shield Association and Blue Health Intelligence, which uses a market-leading claims database to uncover key trends and insights into healthcare affordability and access to care. Analysis was performed by and also includes medical claims data from HealthCore, a wholly owned and independently operated health outcomes subsidiary of Anthem, Inc.
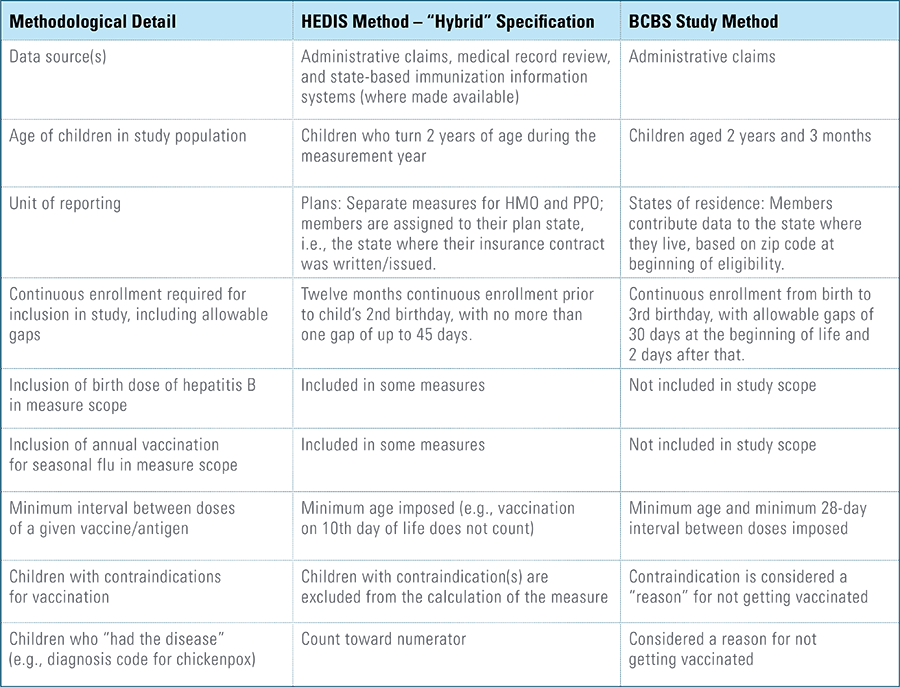
This study looks at medical claims for services taking place between January 1, 2010 through December 31, 2016. Children with three years of continuous medical eligibility in a commercial BCBS plan were included in the study as summarized below:

Total sample size (the number of children contributing data to the childhood vaccination rates) was 843,610 children.
For the children in this study, each birth cohort is labeled by the year in which the child is born, followed by data for three years for each cohort. As a result, for children born in 2010, data from 2010, 2011, 2012 and 2013 are included; for children born in 2011, data from 2011, 2012, 2013 and 2014 are included, and so on for the next two birth years.
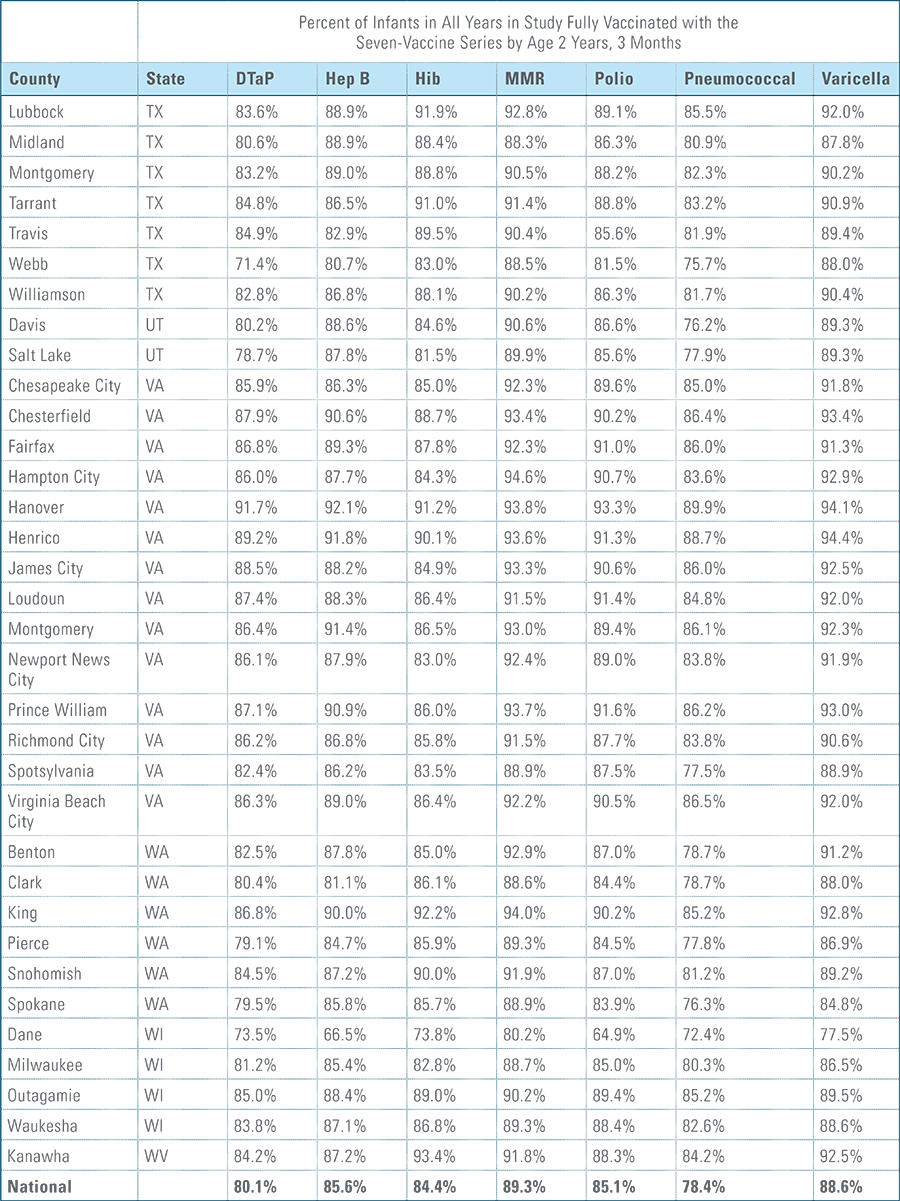
Children are counted as fully immunized with the series or individual vaccines as long as they receive the needed vaccine(s) by age 27 months, allowing nine months of buffer time for parents/guardians running behind schedule to take their children to their physician to receive their vaccines.
The report does not capture members who will receive the childhood vaccines after 27 months of age or members who received vaccines from a free public or private local program that does not generate a medical claim.
For more information and to read past reports from The Health of America Report series, visit www.bcbs.com/the-health-of-america.
Methodological Notes
- The analysis included all medical claims for vaccine utilization.
- Metrics were adjusted for presence of “administration-only” vaccination claims.
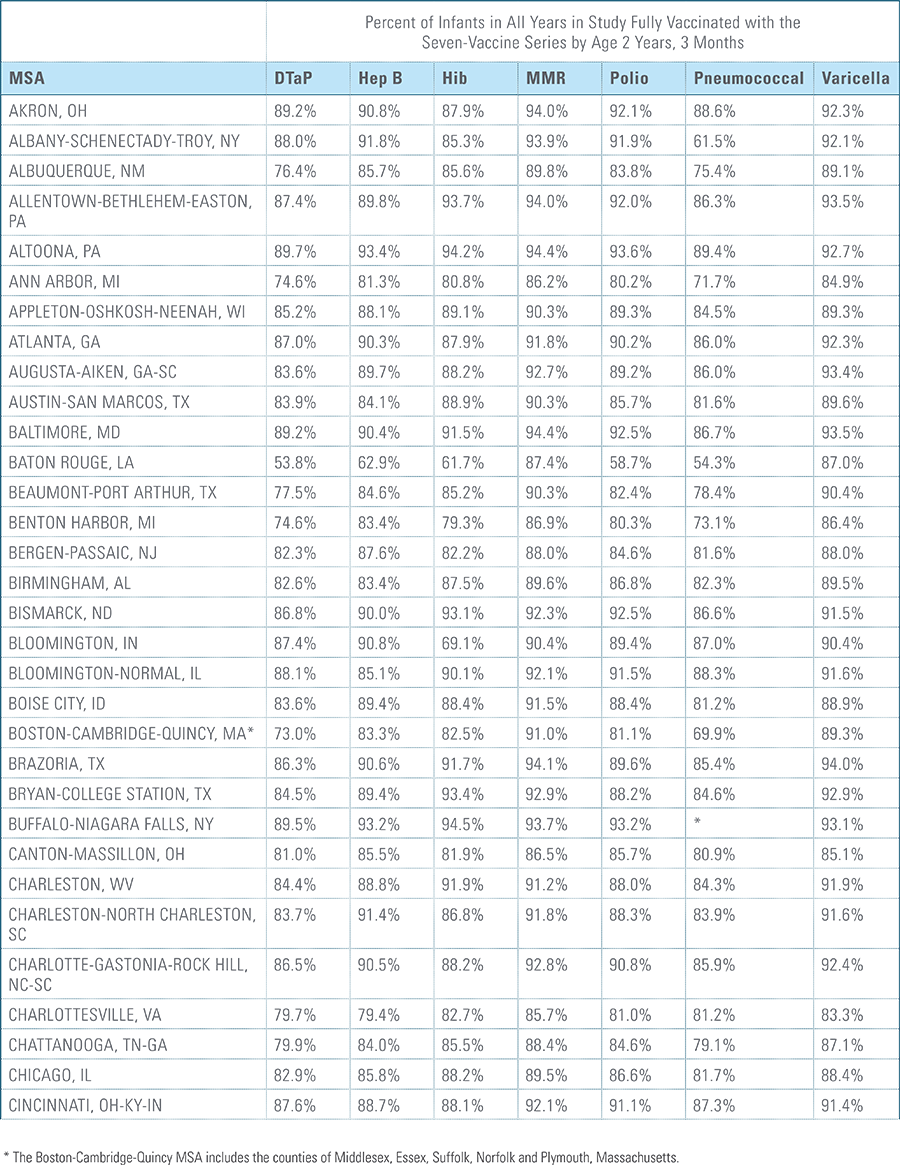
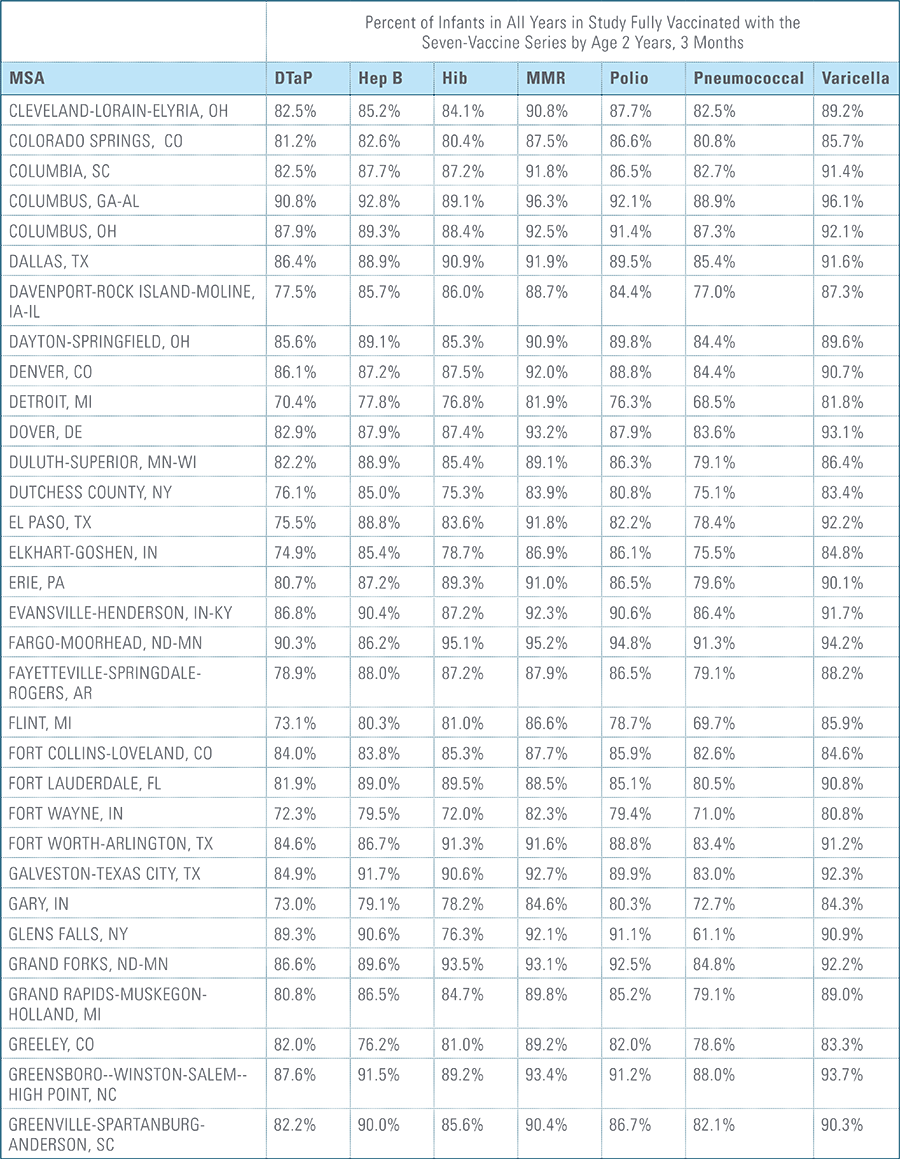
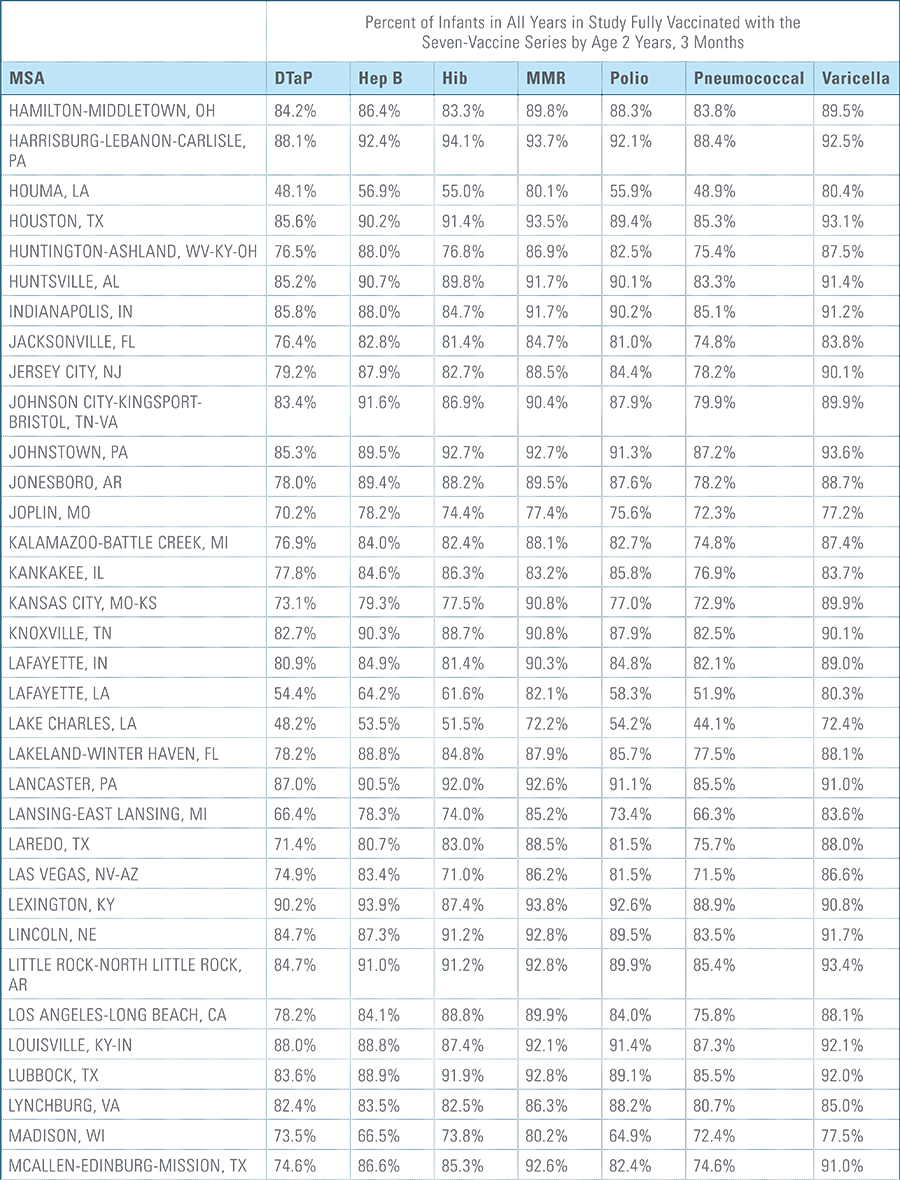
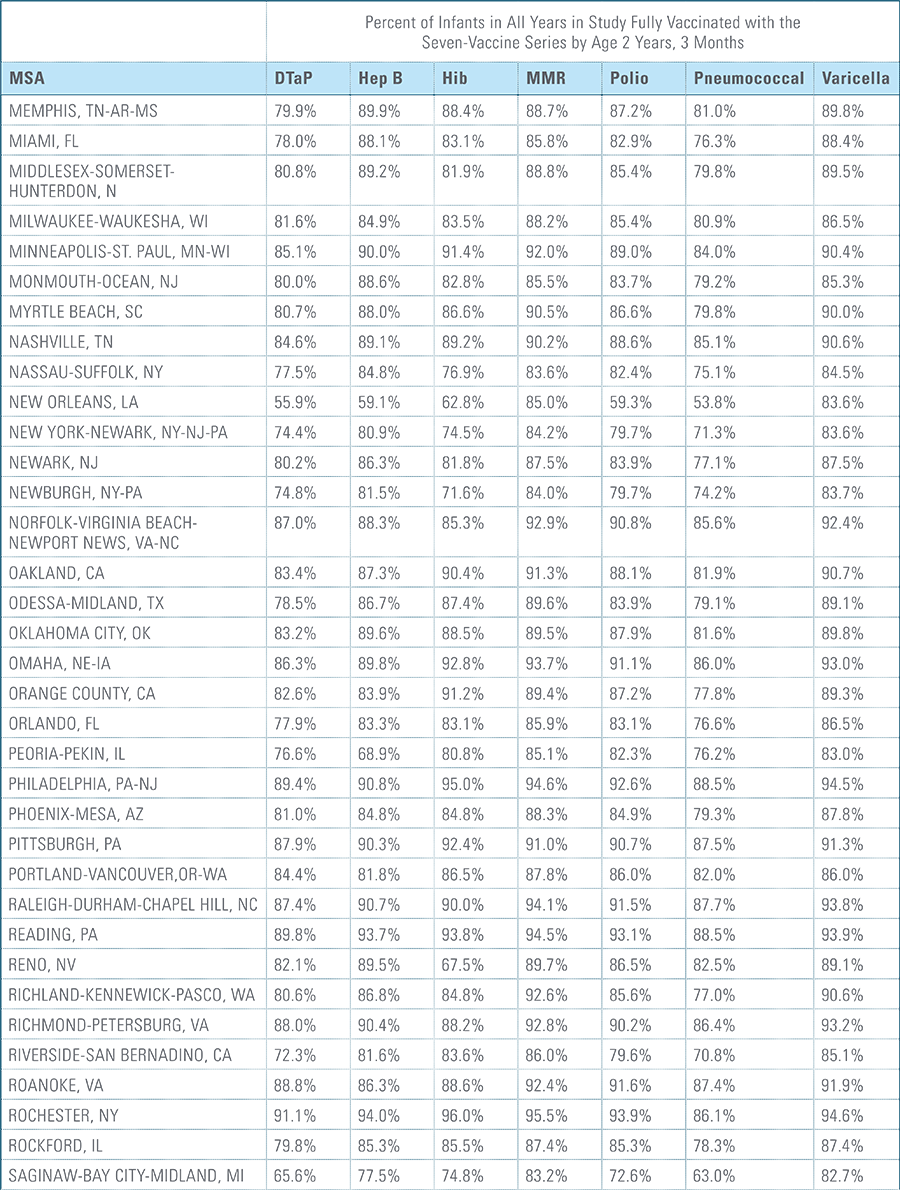
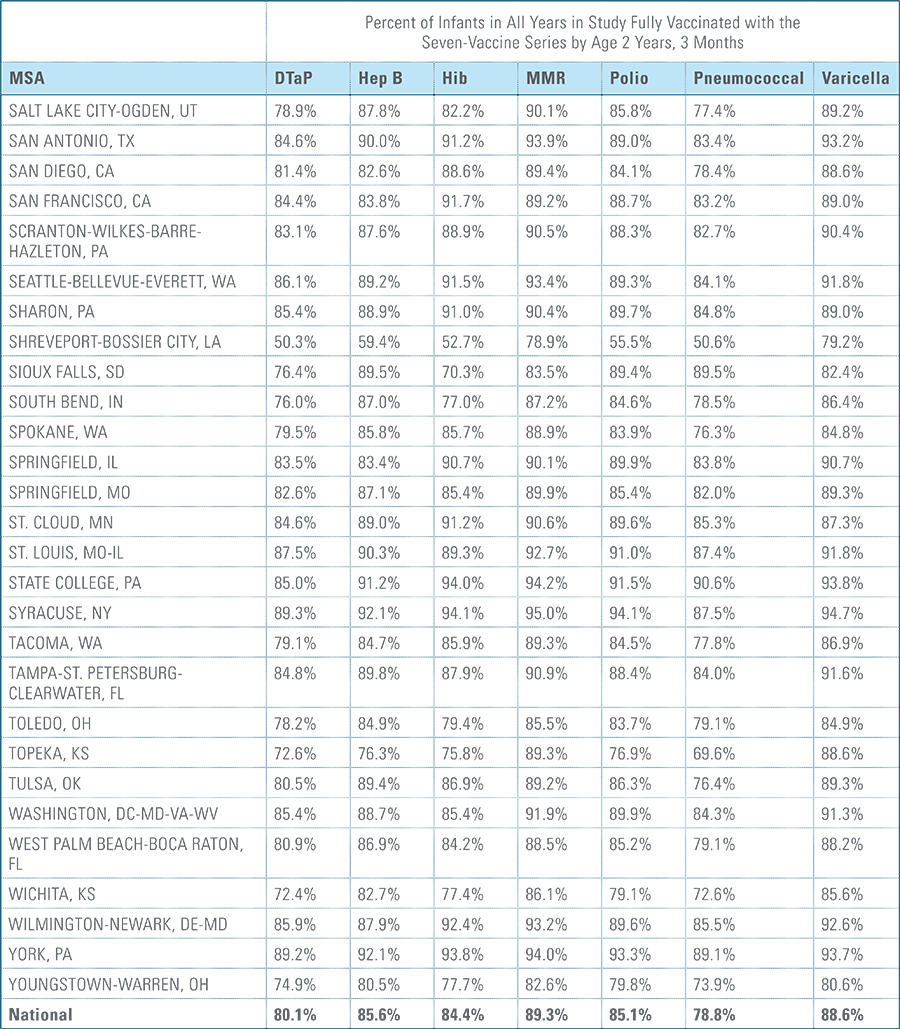
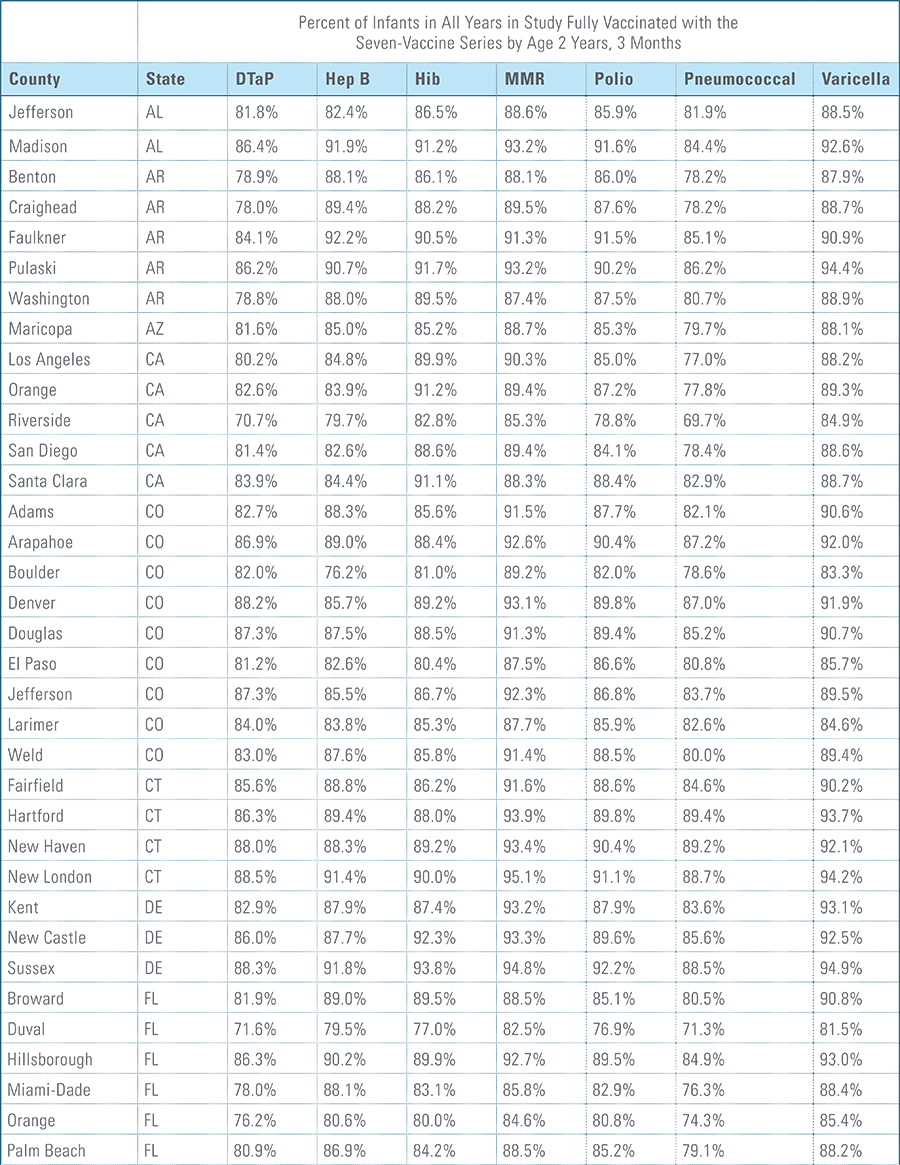
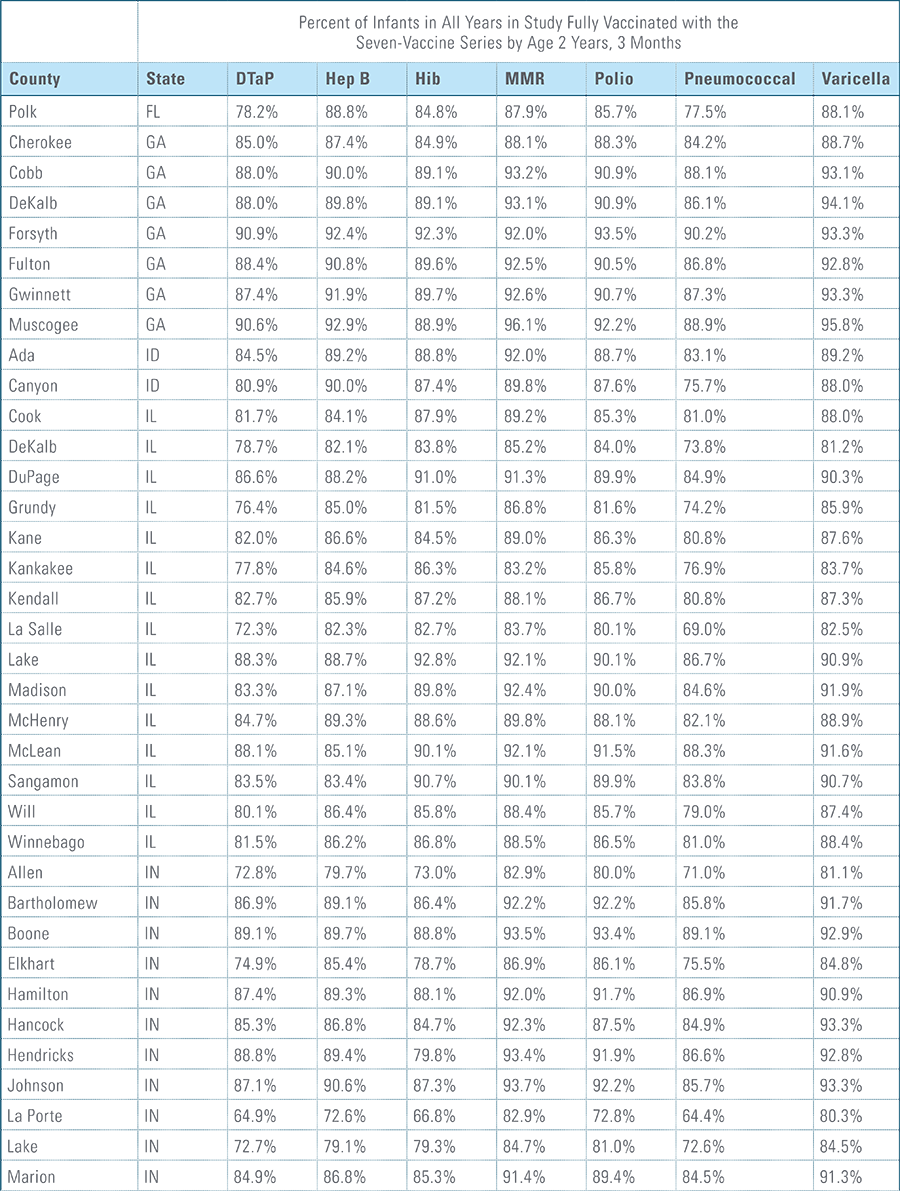
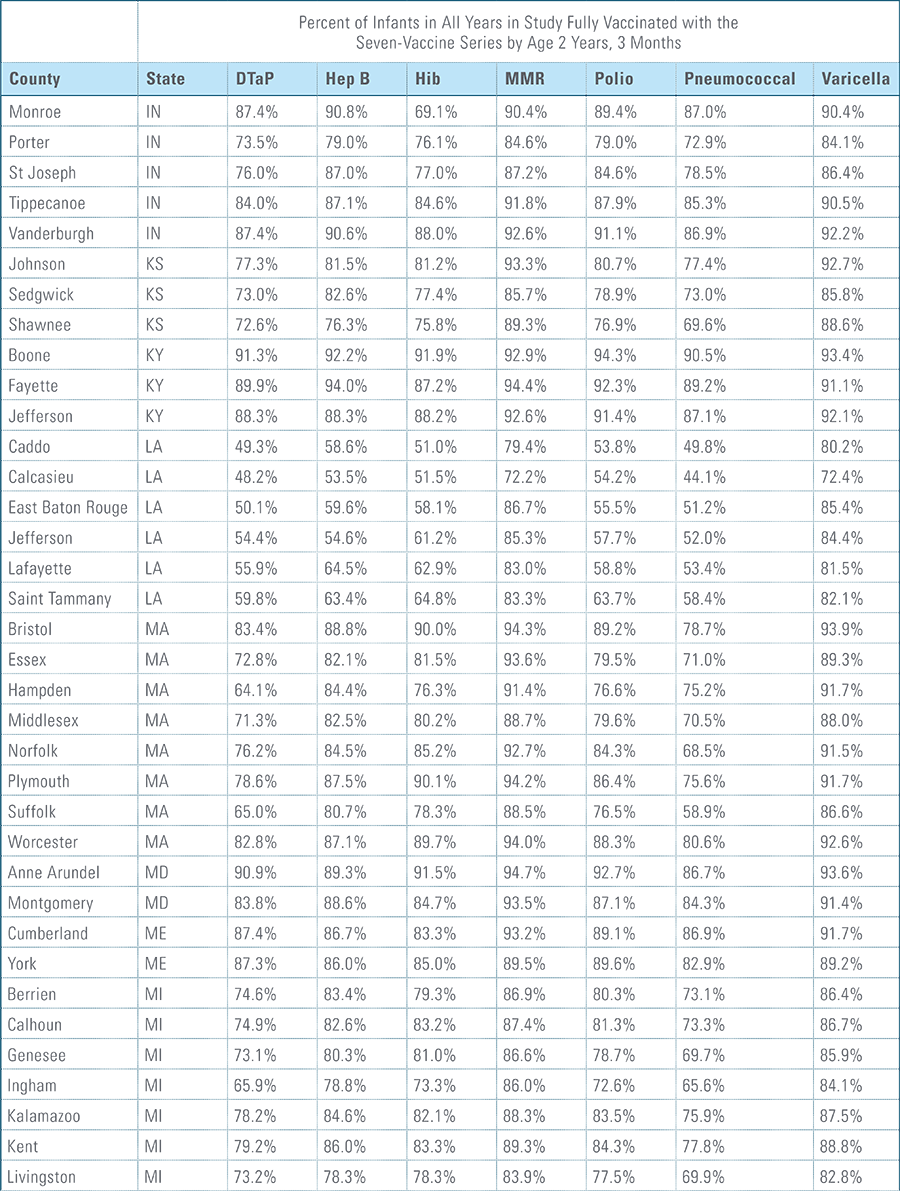
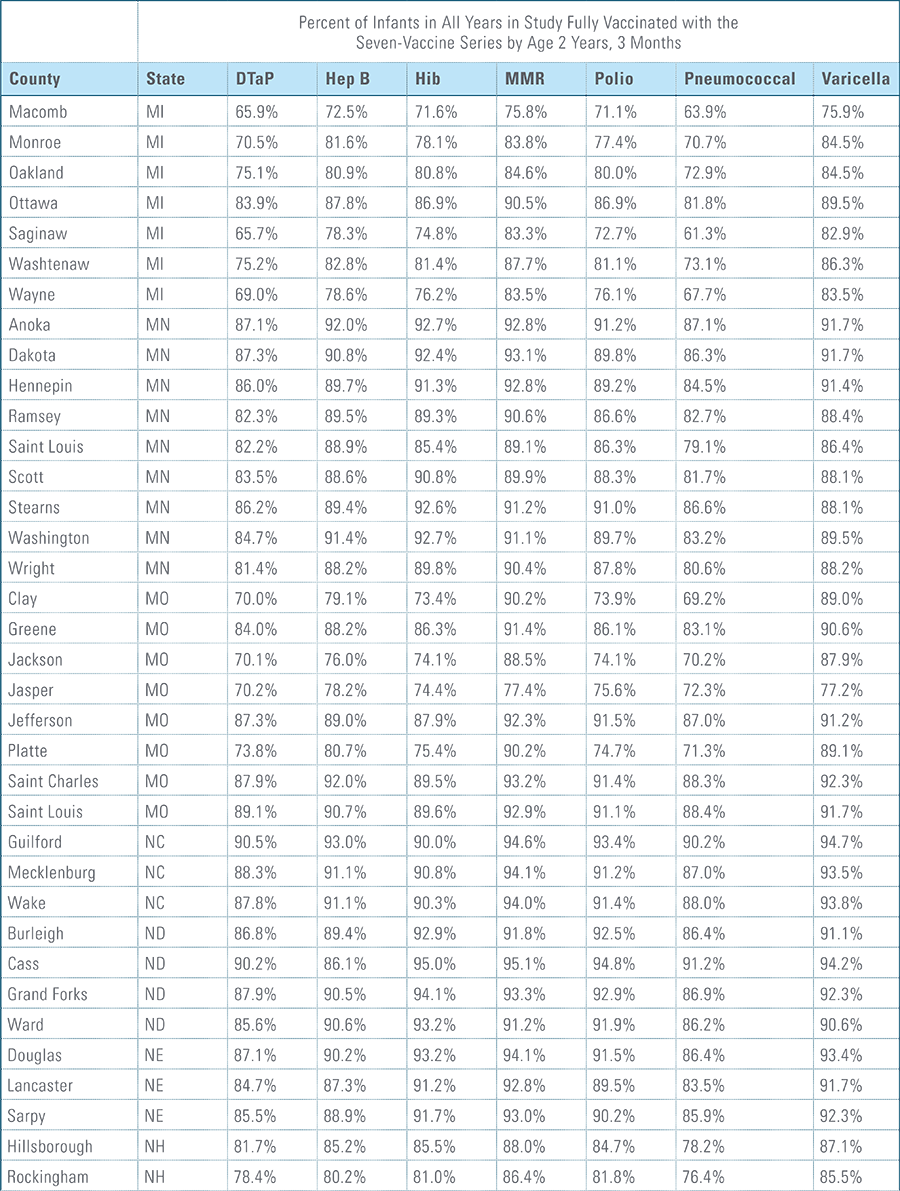
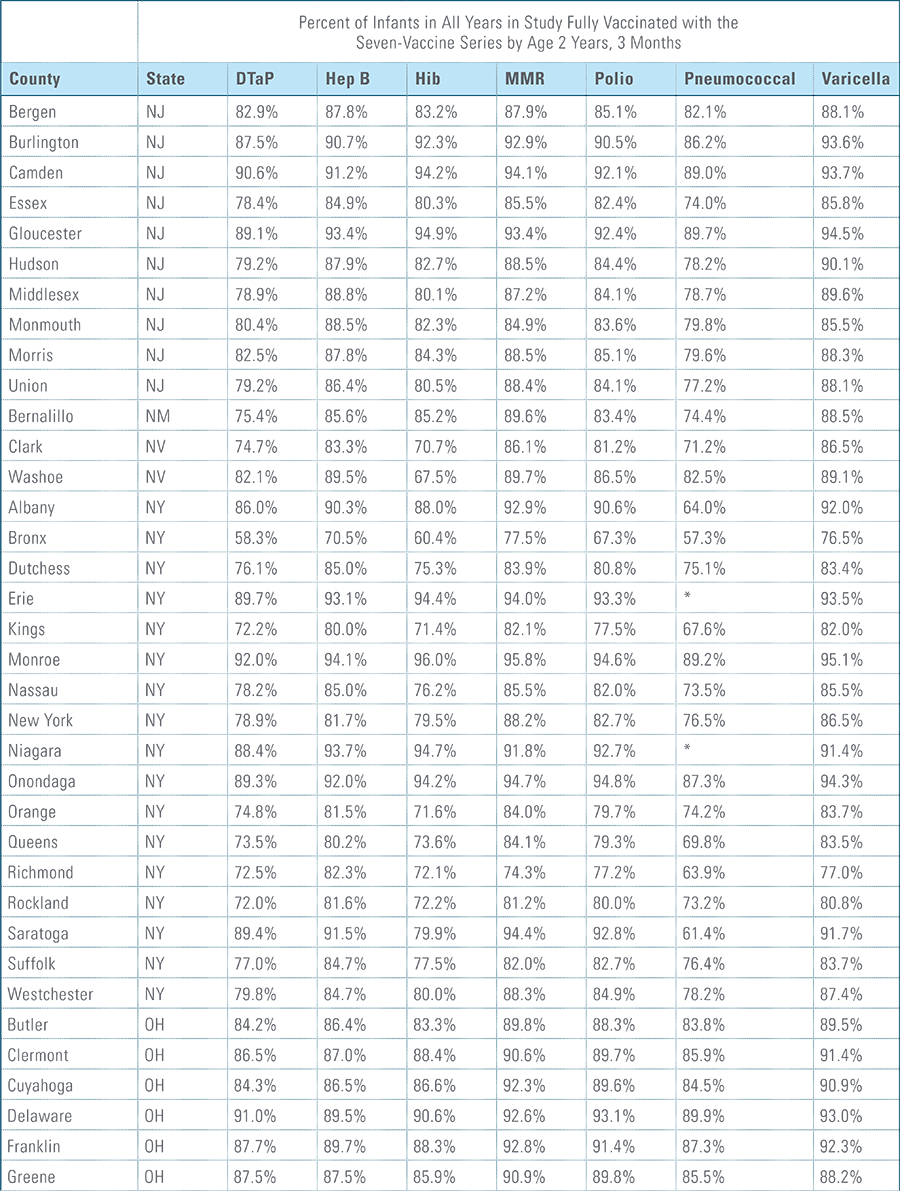
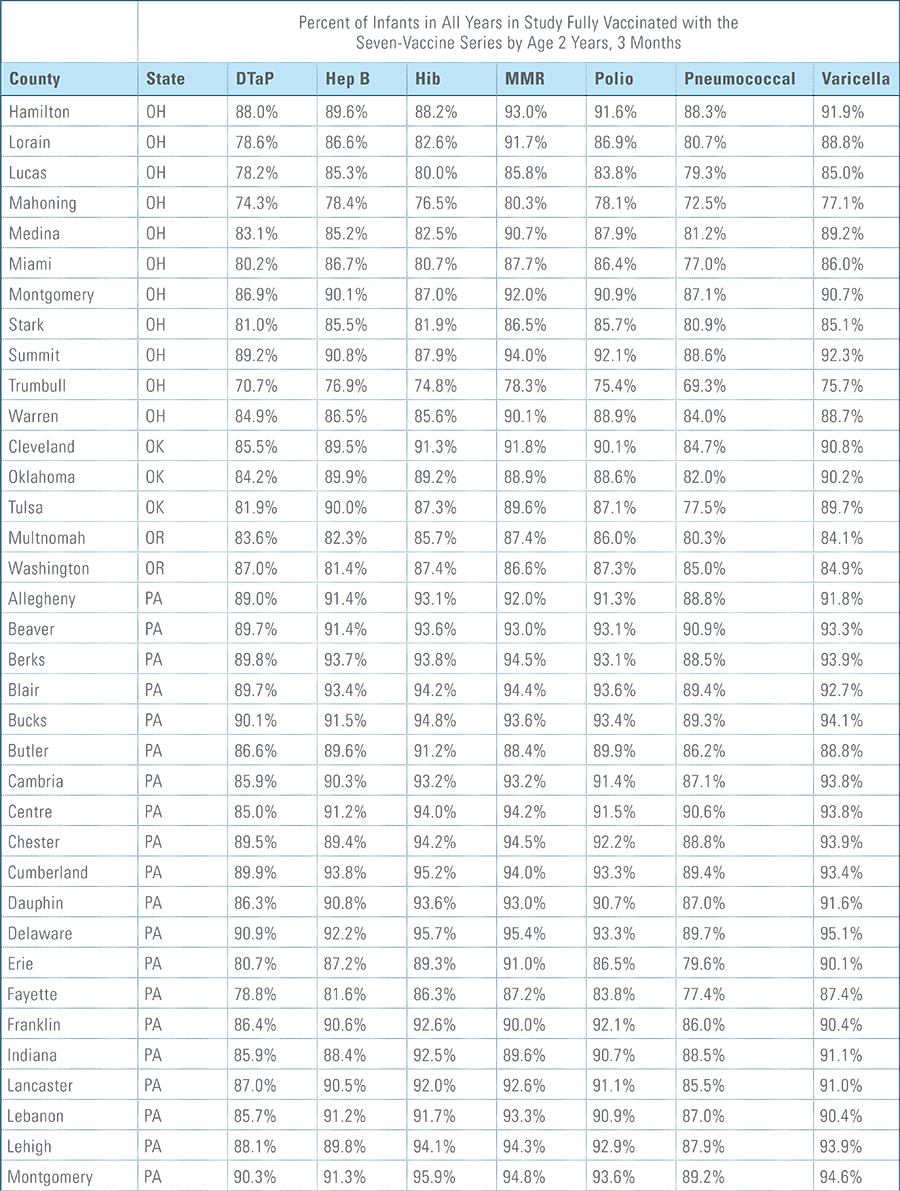
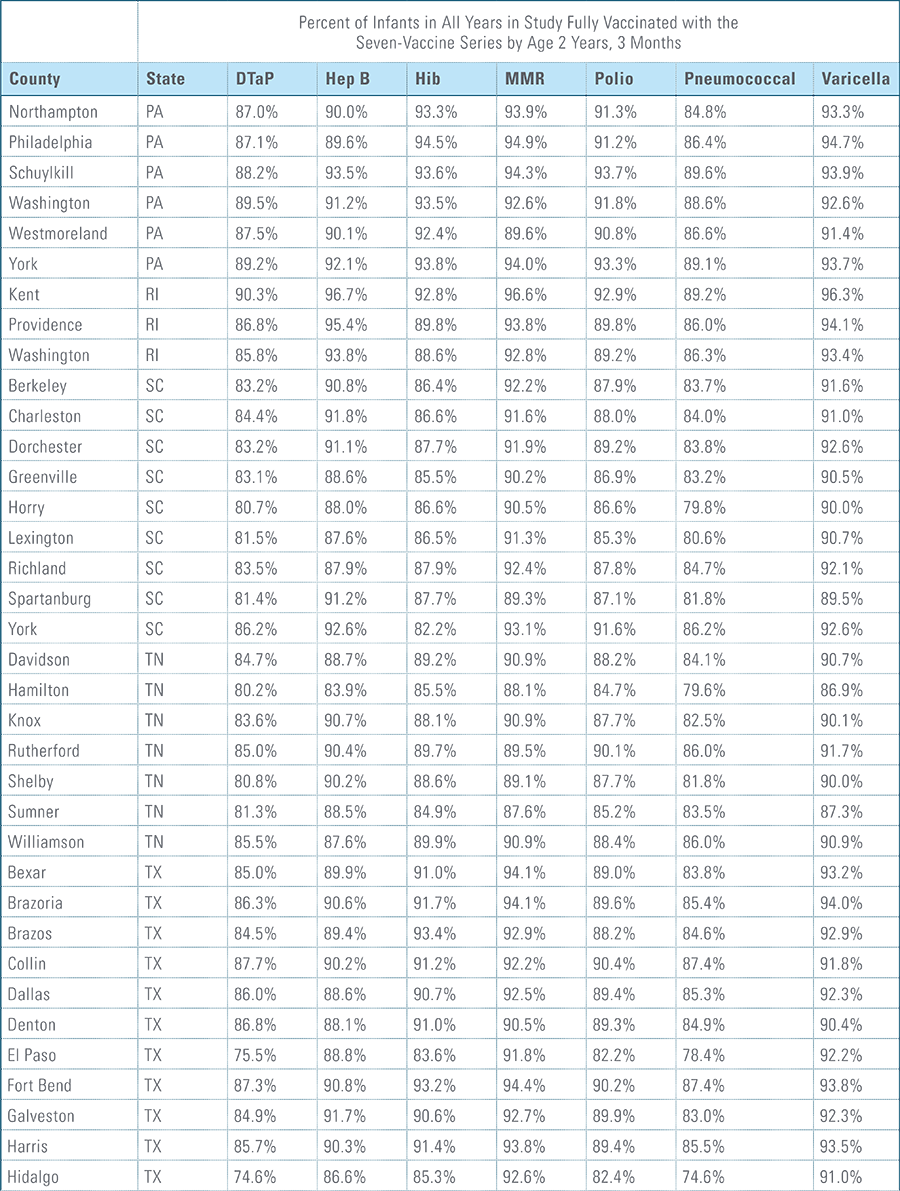
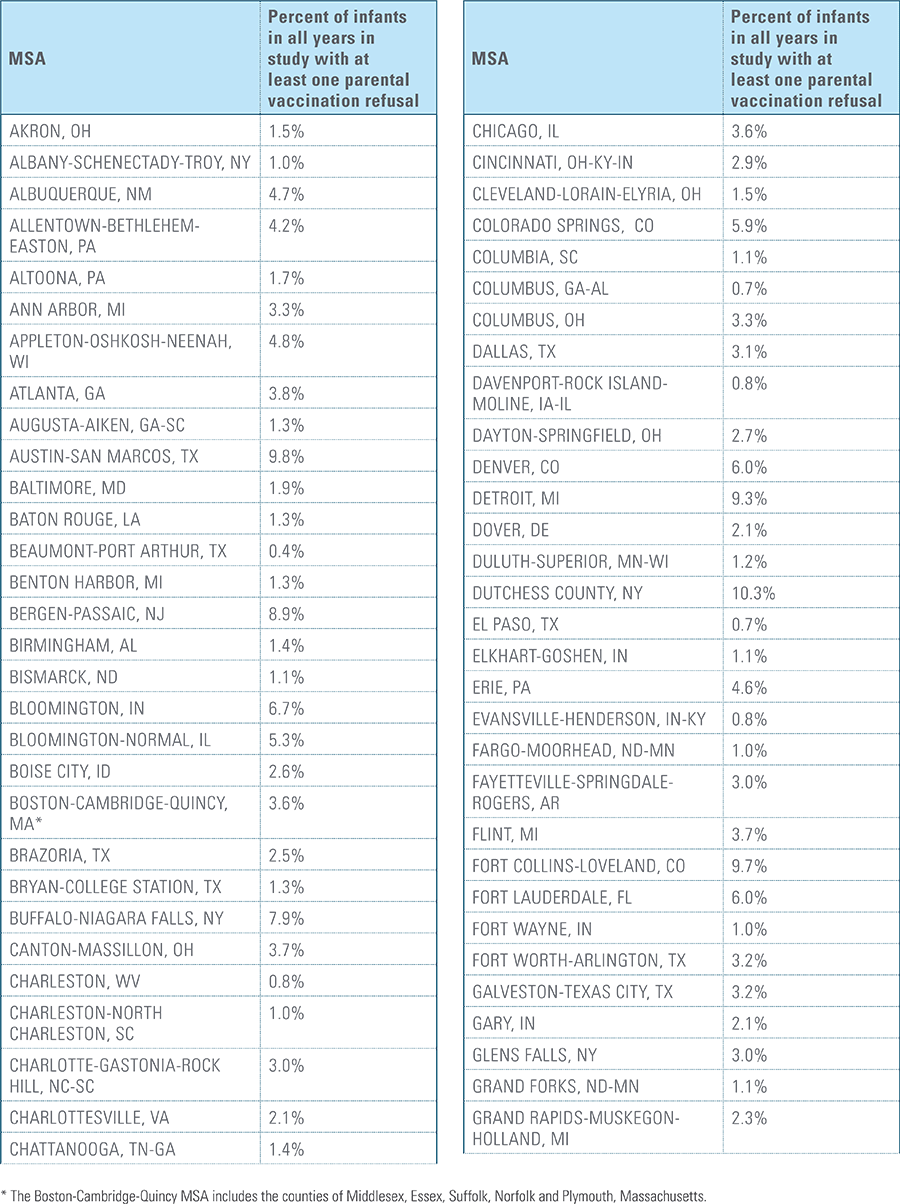
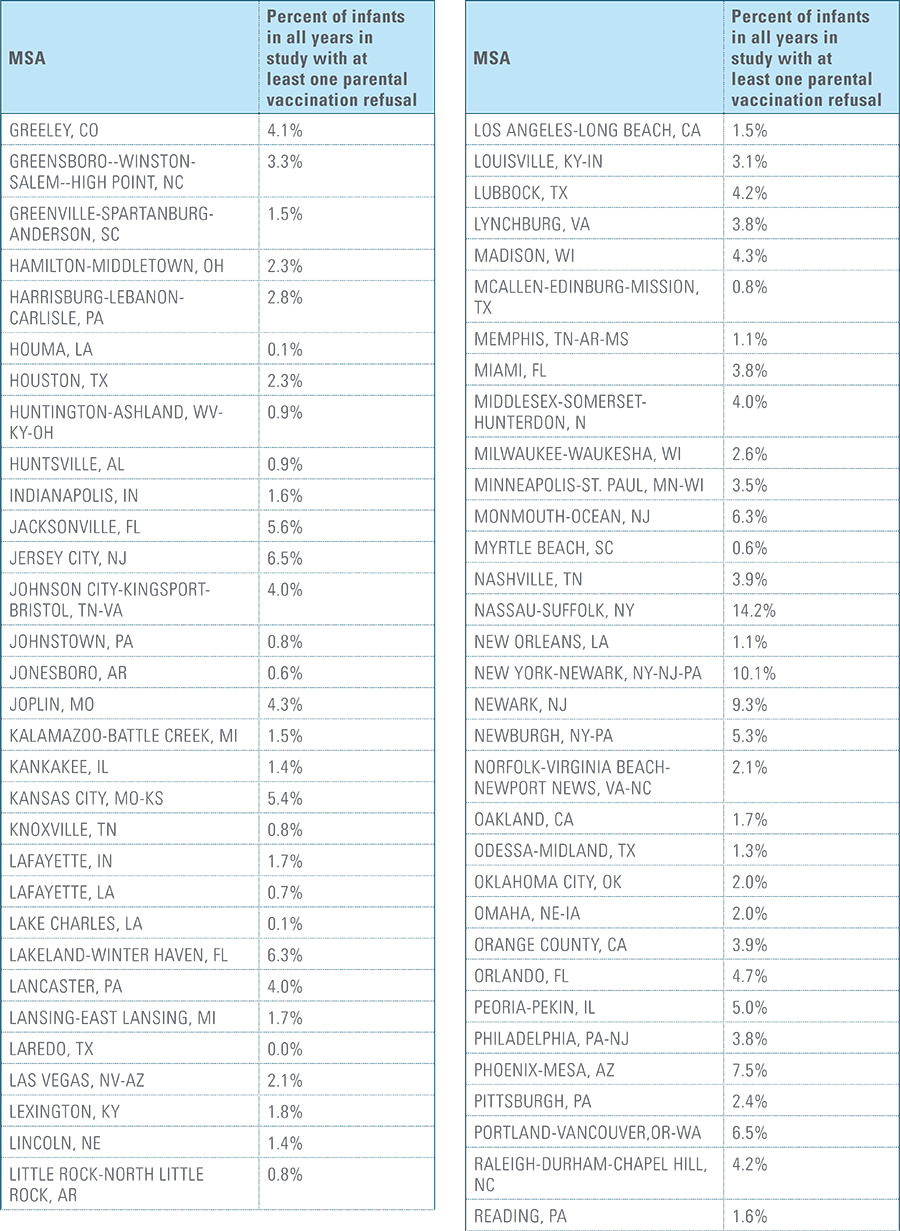
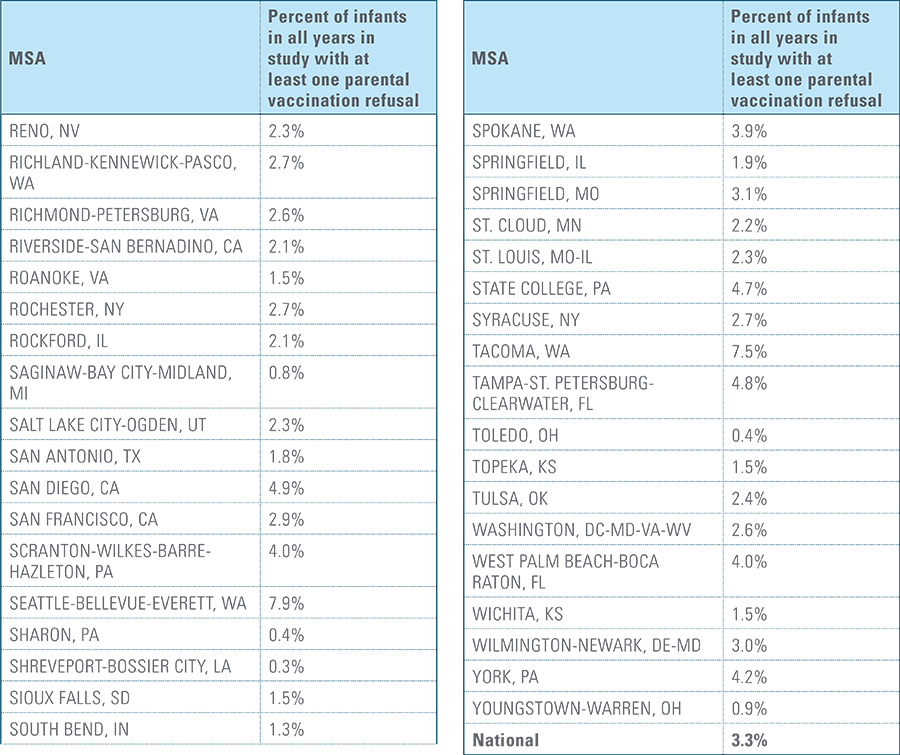
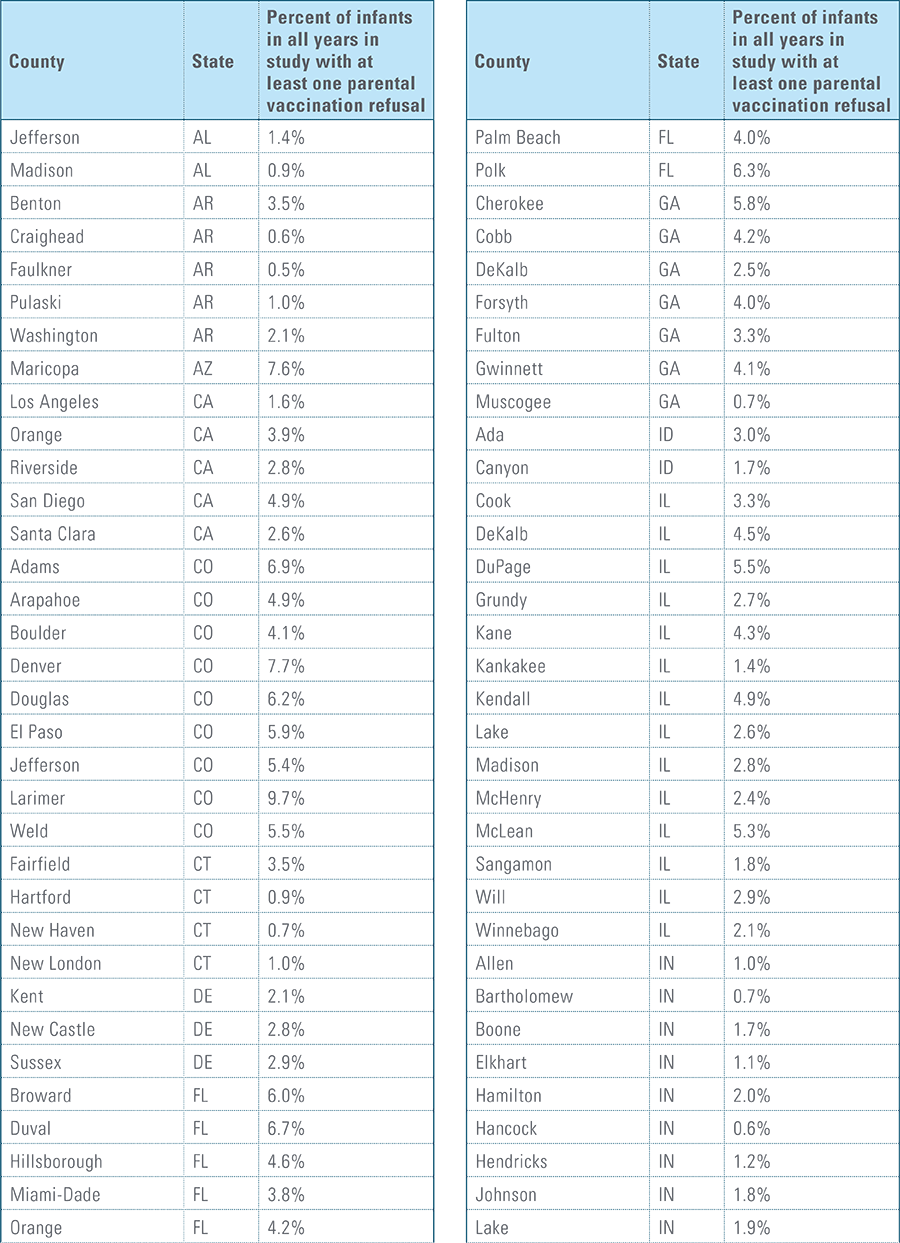
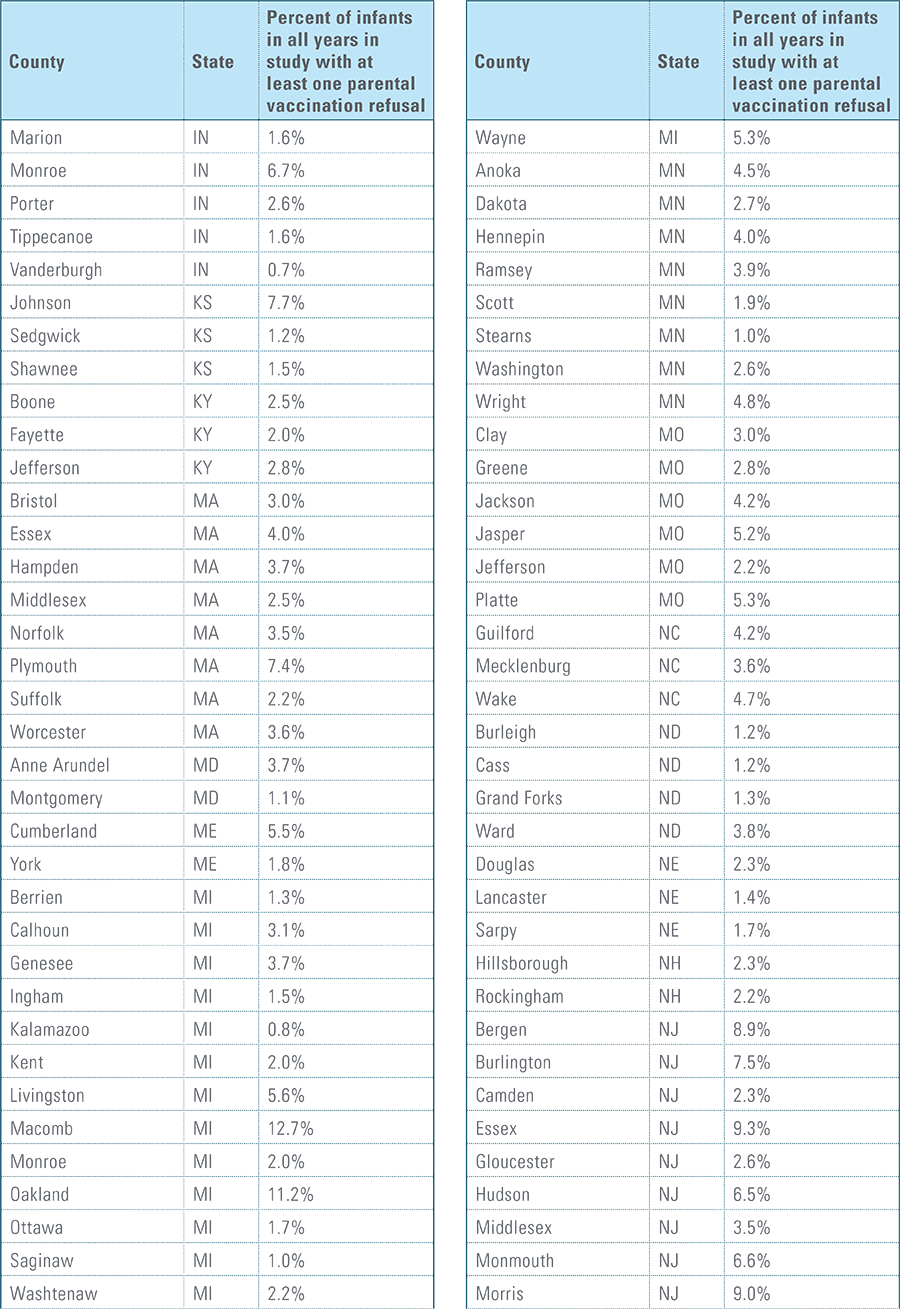
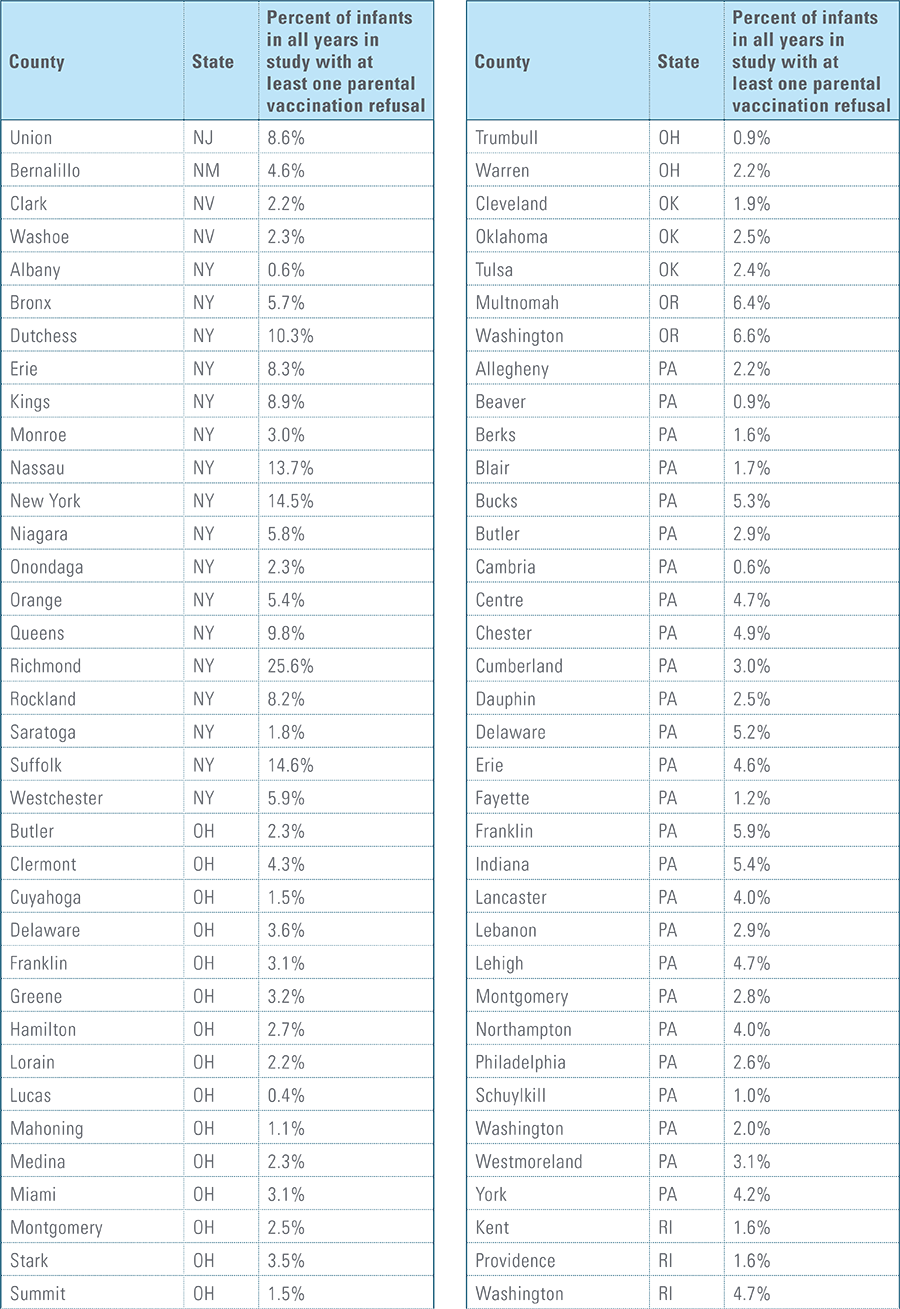
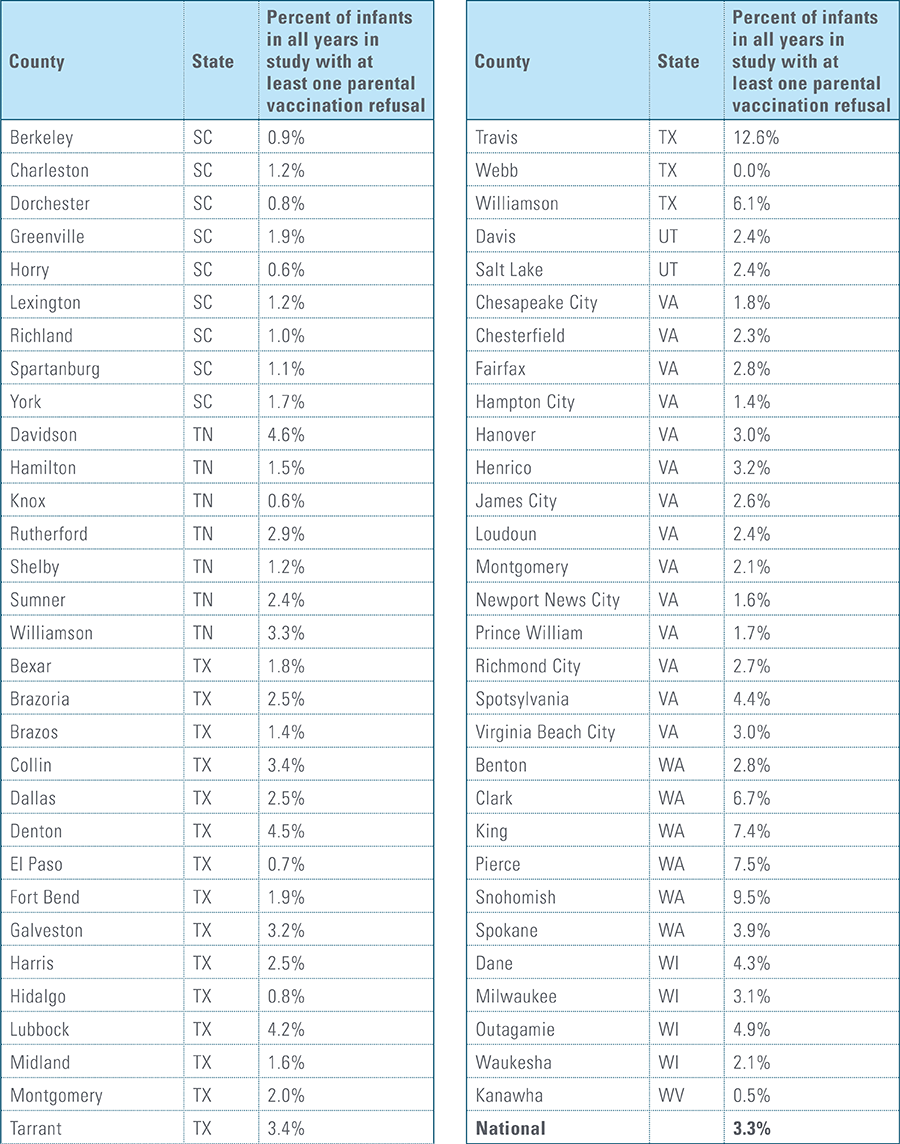
- States, metropolitan statistical areas and counties with less than 500 children in a given sample were excluded from display.
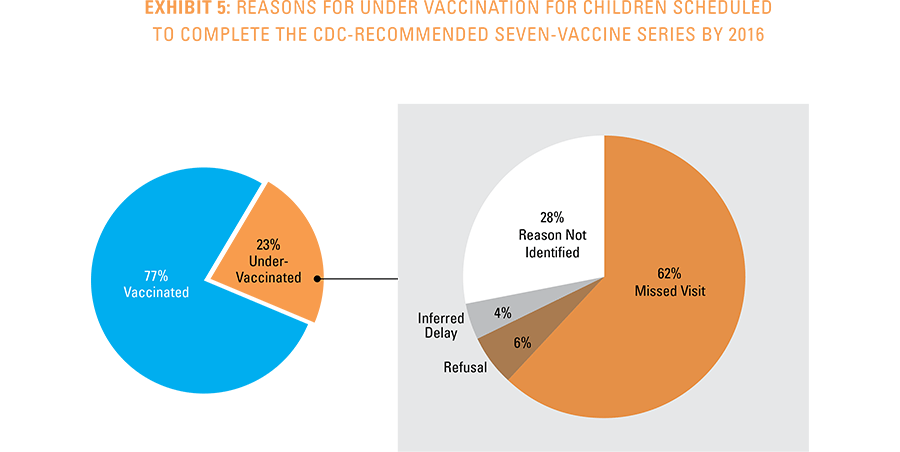
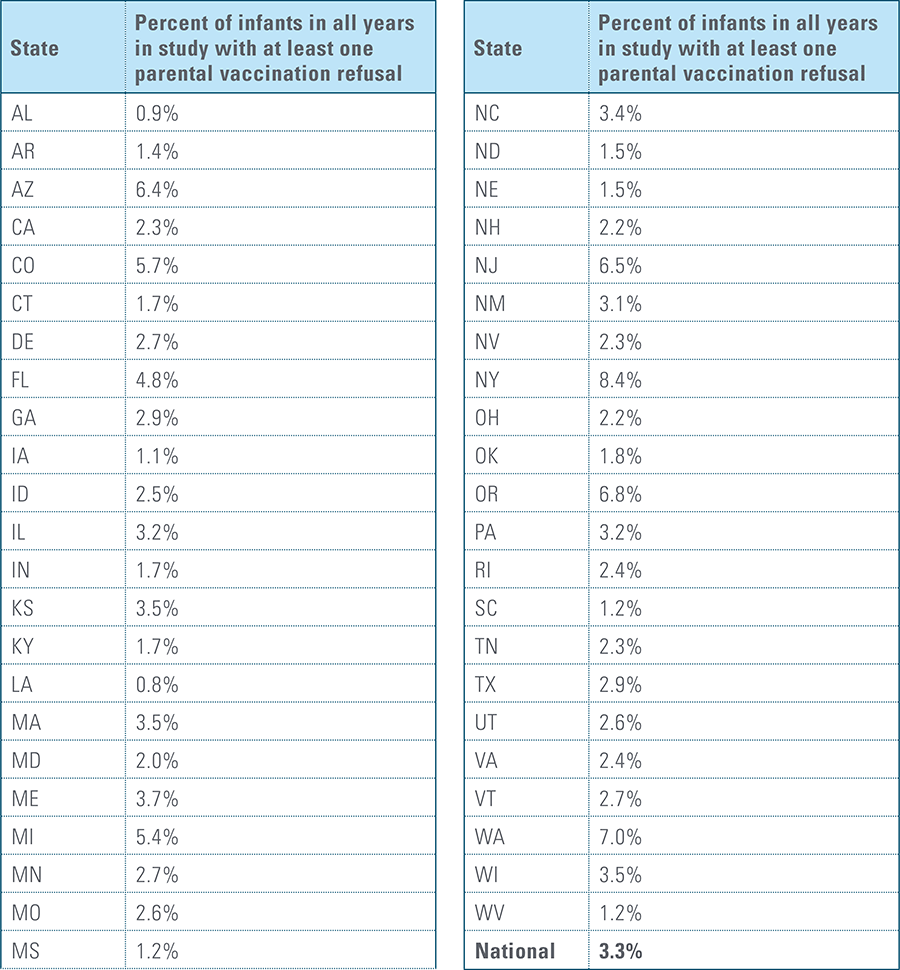
Under Vaccination Rationale Hierarchy
A reason for under vaccination was assigned for each insufficiently vaccinated case based on information
available in the claims data and following the order indicated below. When more than one reason is identified
for a given child, the highest ranked item below was taken as the reason for under vaccination:
- Child had a contraindication for vaccination, for example HIV.
- Child’s parent/guardian refused at least one vaccine as identifiable by diagnosis code.
- Child had the disease the vaccine is meant to prevent.
- For example: A diagnosis code for varicella (chicken pox) would be a “reason” for non-compliance with the varicella vaccine.
- Presence of disease was assessed from birth to age 2 years and 3 months.
- Child missed at least two well-child visits to a physician.
- Number of well-child visits up to age 2 years and 3 months was less than nine visits. (AAP recommends 10 well-child visits by this age.)
- Inferred delay
- Child was not up-to-date as of age 2 years and 3 months but was up-to-date as of age 3 years.
- Unknown reason
Data Exclusion
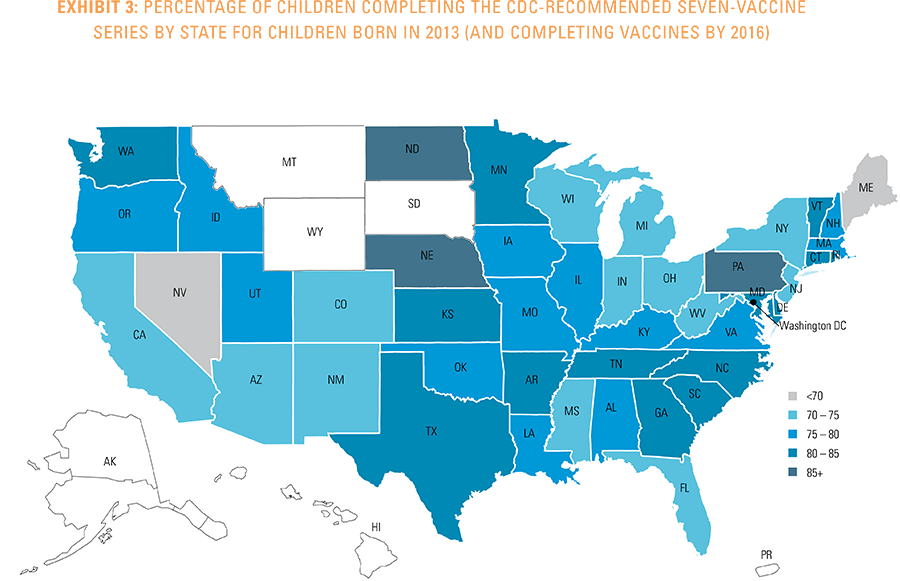
Data from Anthem BCBS were excluded from California because of the prevalence of capitated payments to primary care providers, reducing the incentive for providers to submit claims for primary care, and under representing vaccination rates in that state. Additionally, in several cases, insufficient sample sizes resulted in the exclusion of certain states or metropolitan statistical areas from the reporting of results. Sample size minimums exclude the reporting of results from Alaska, Hawaii, Montana, South Dakota, Washington D.C. and Wyoming and select metropolitan areas.
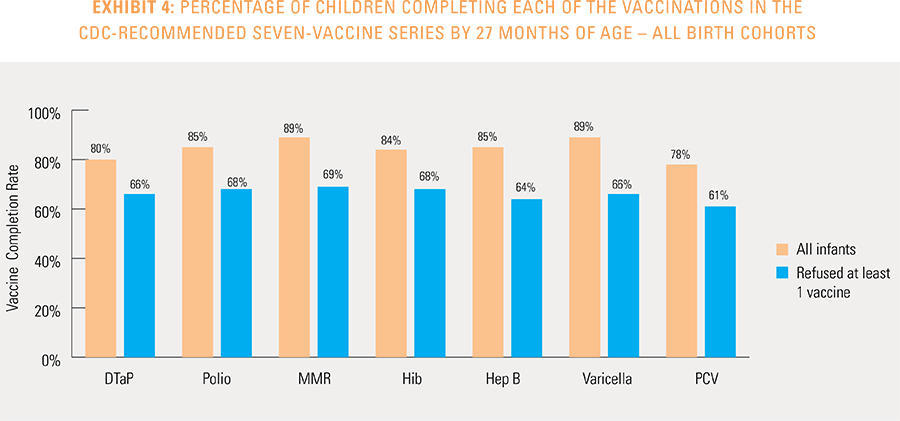
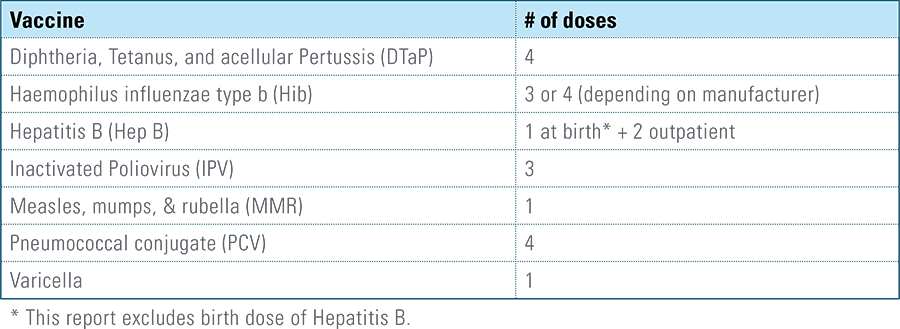
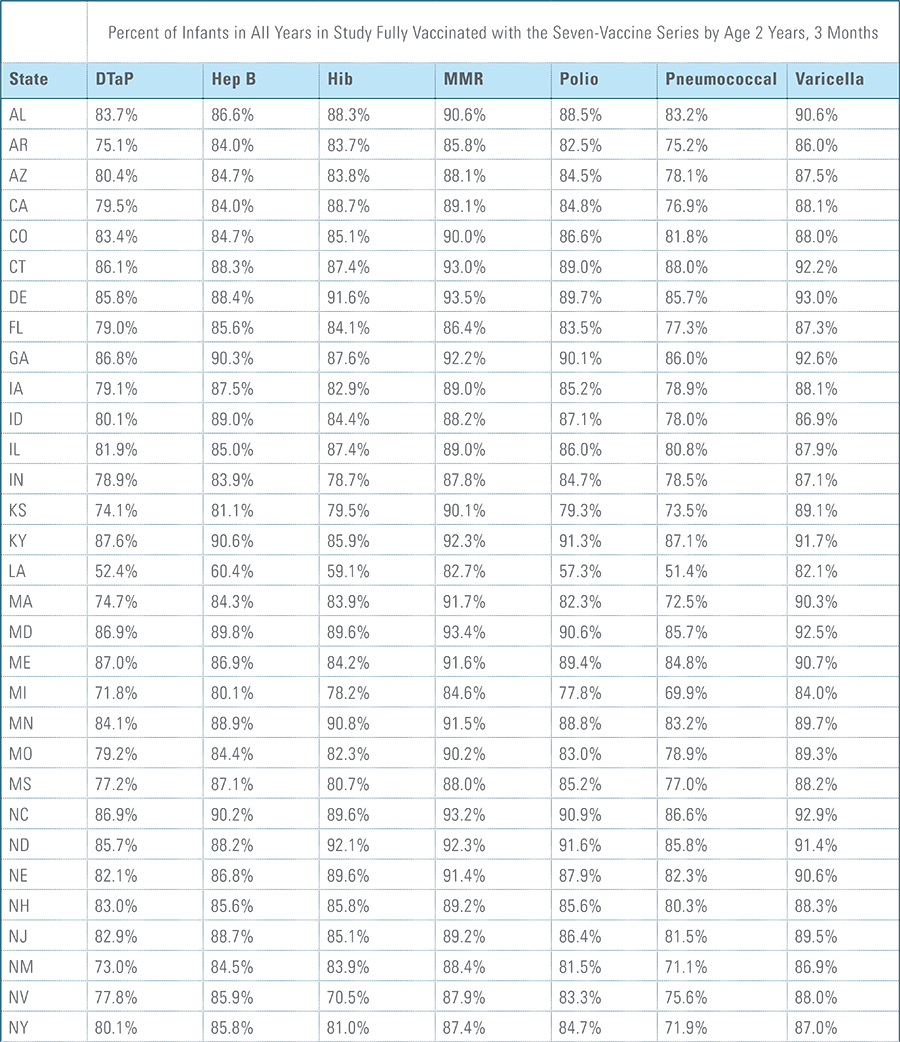
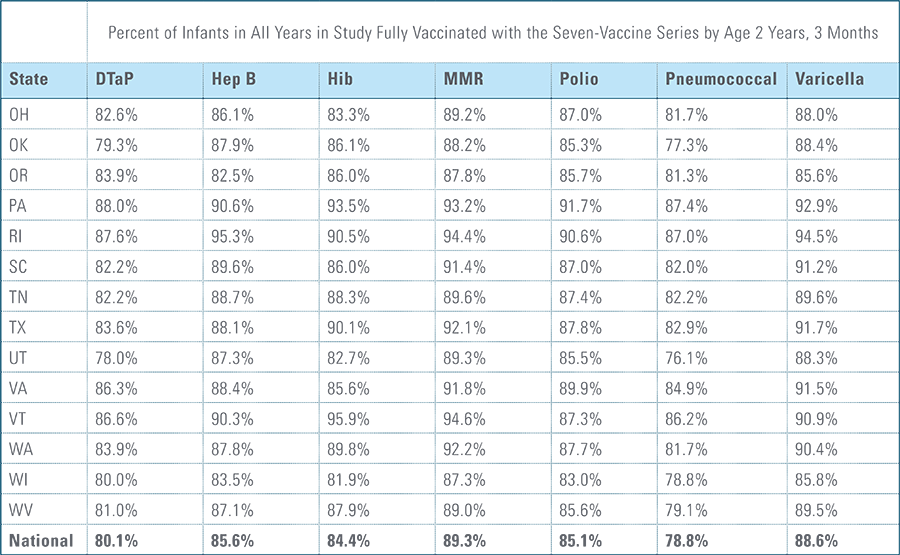
 Childhood vaccination is considered one of the top public health accomplishments of the 20th century.1,2 In this new report, the Blue Cross Blue Shield Association, in partnership with HealthCore and Blue Health Intelligence (BHI), examines early childhood vaccination trends for U.S. commercially insured children. The report looks at claims data for children in four birth cohorts (those born in 2010, 2011, 2012 and 2013) and follows their care continuously from birth through 3 years of age.3 This study focuses on completion rates of vaccines in the Centers for Disease Control and Prevention’s recommended seven-vaccine series for children between the years 2013 (by which time the vaccines should have been completed for the first birth cohort) and 2016.4,5,6 (See Appendix A for details on the seven-vaccine series and other CDC-recommended vaccinations and Appendices B and C for specific study methodology.)
Childhood vaccination is considered one of the top public health accomplishments of the 20th century.1,2 In this new report, the Blue Cross Blue Shield Association, in partnership with HealthCore and Blue Health Intelligence (BHI), examines early childhood vaccination trends for U.S. commercially insured children. The report looks at claims data for children in four birth cohorts (those born in 2010, 2011, 2012 and 2013) and follows their care continuously from birth through 3 years of age.3 This study focuses on completion rates of vaccines in the Centers for Disease Control and Prevention’s recommended seven-vaccine series for children between the years 2013 (by which time the vaccines should have been completed for the first birth cohort) and 2016.4,5,6 (See Appendix A for details on the seven-vaccine series and other CDC-recommended vaccinations and Appendices B and C for specific study methodology.)


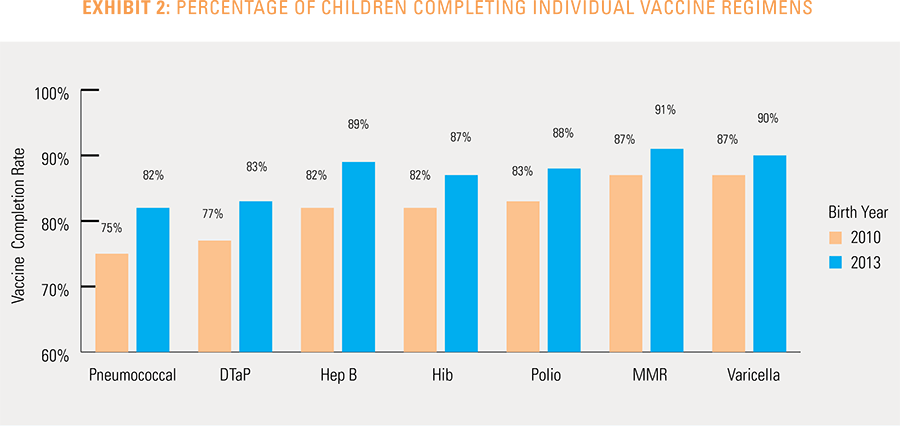
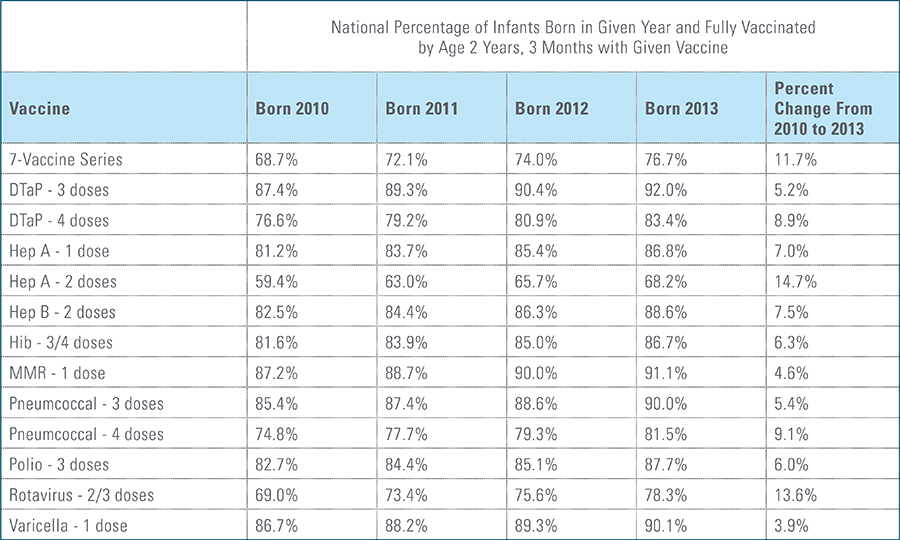
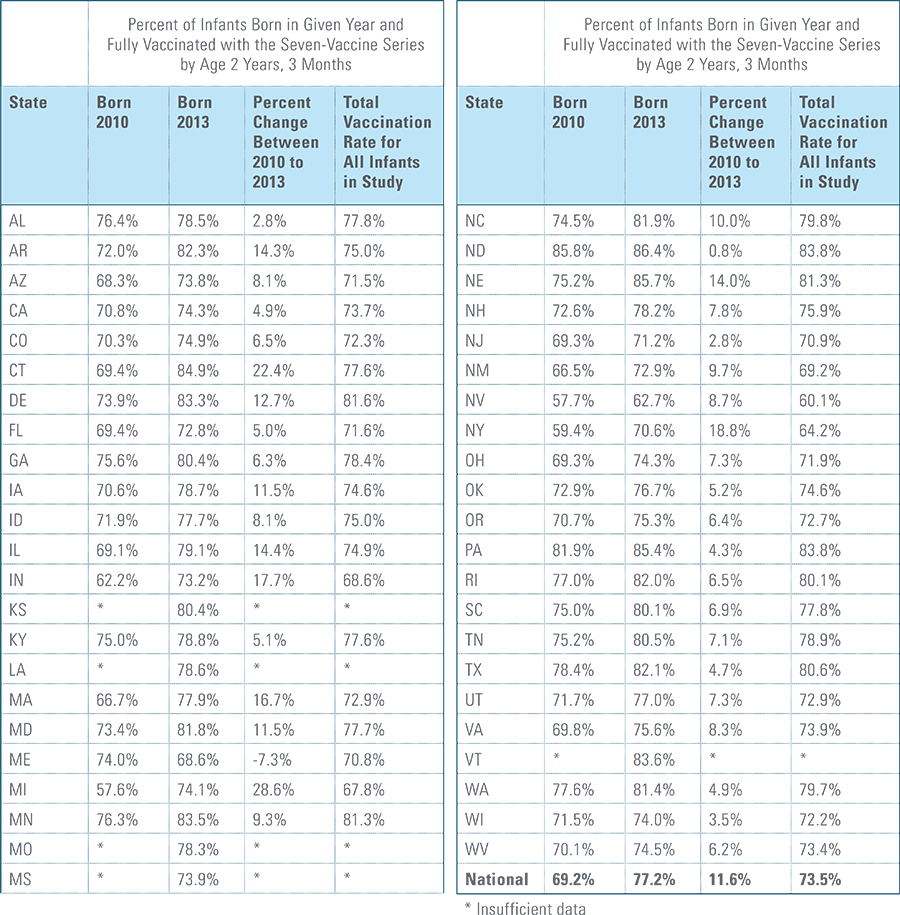
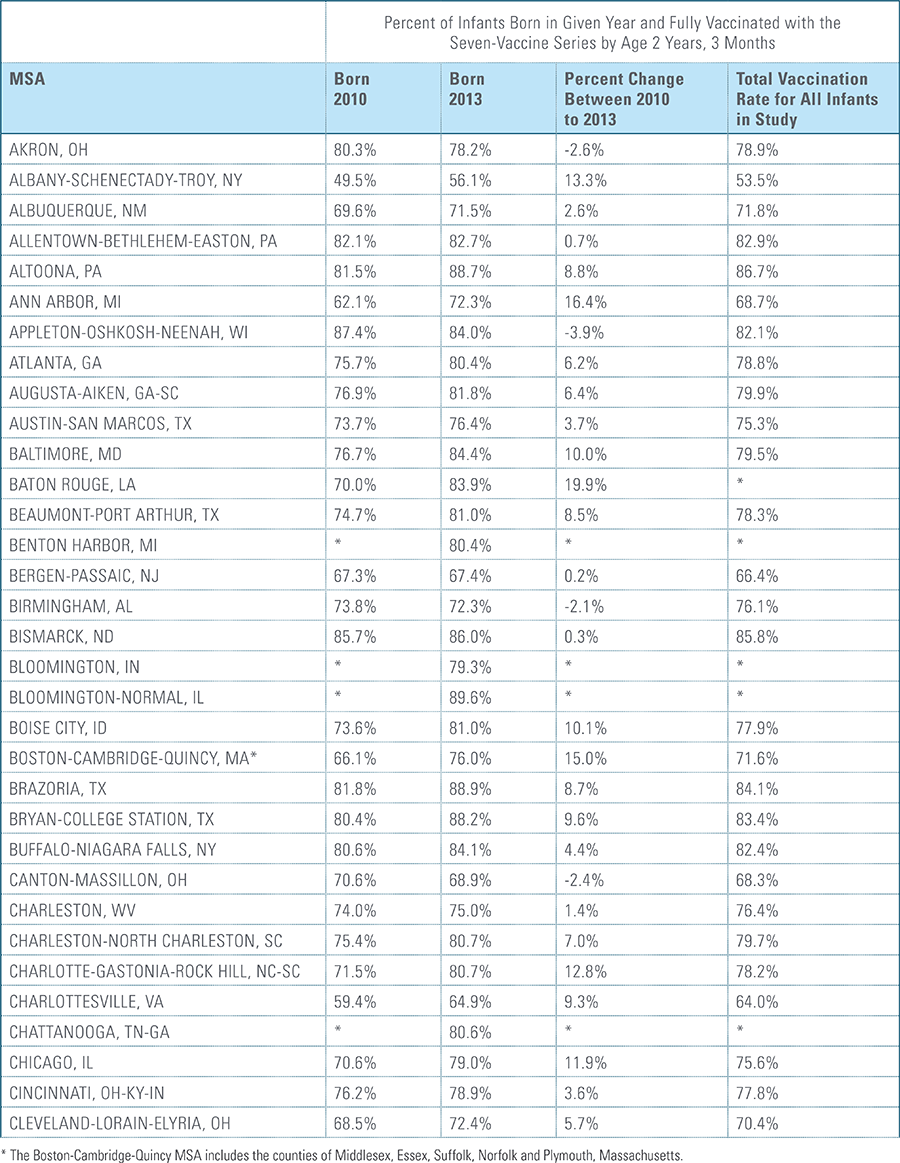
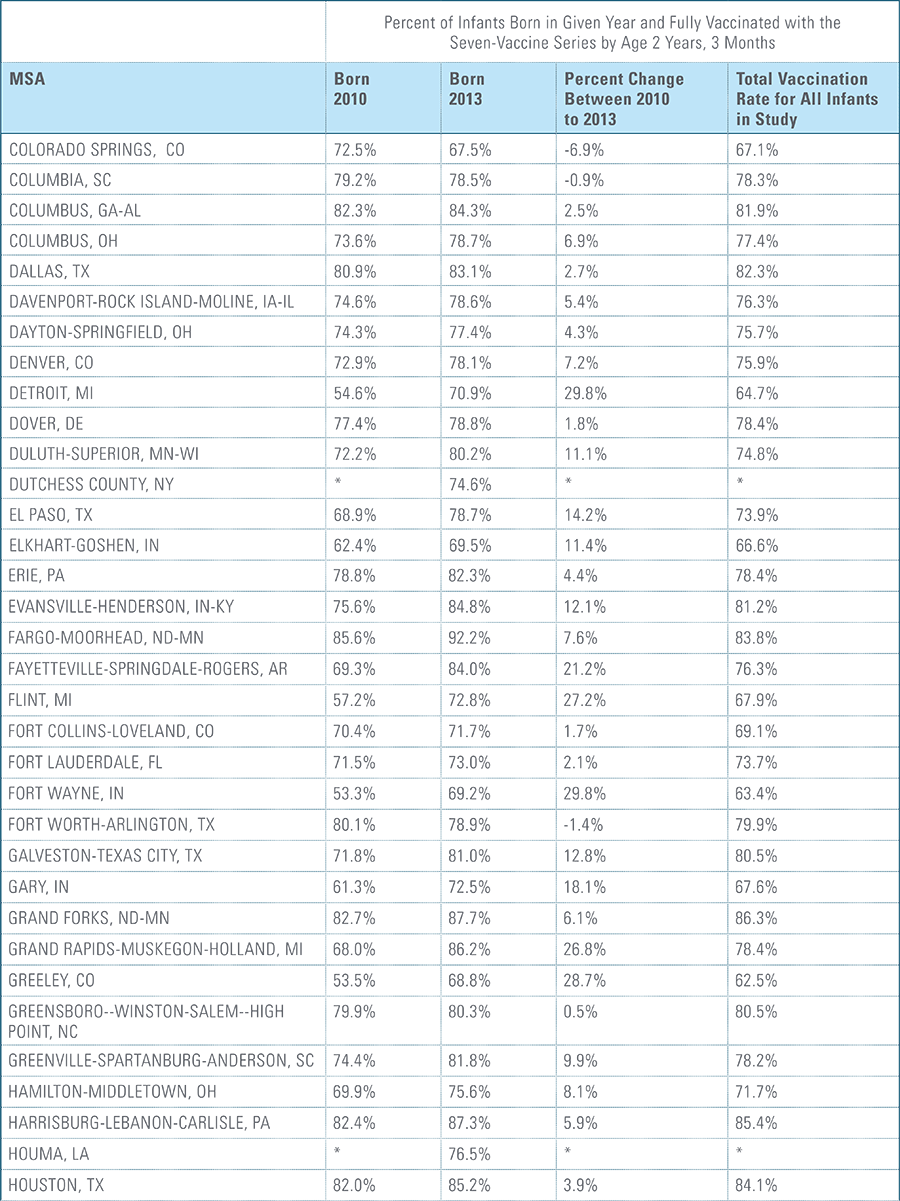
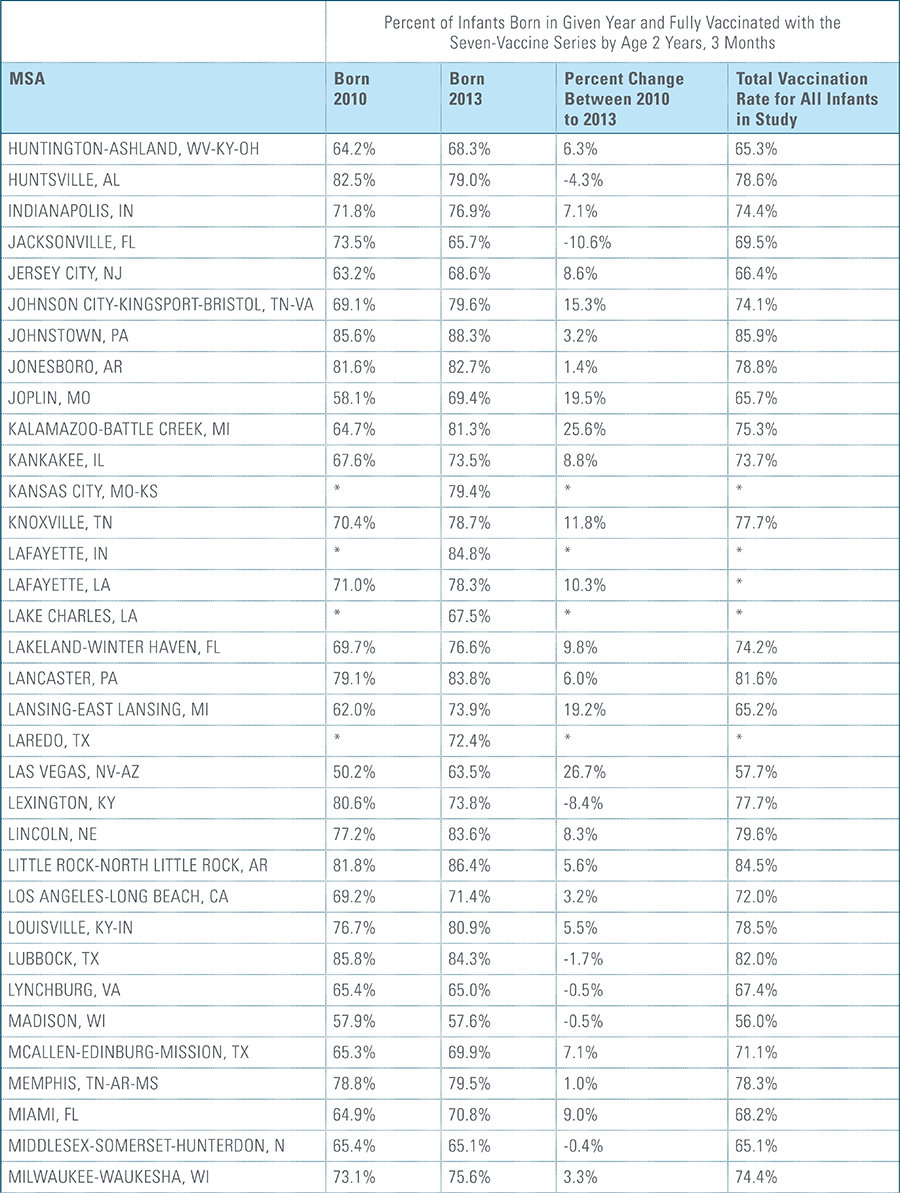
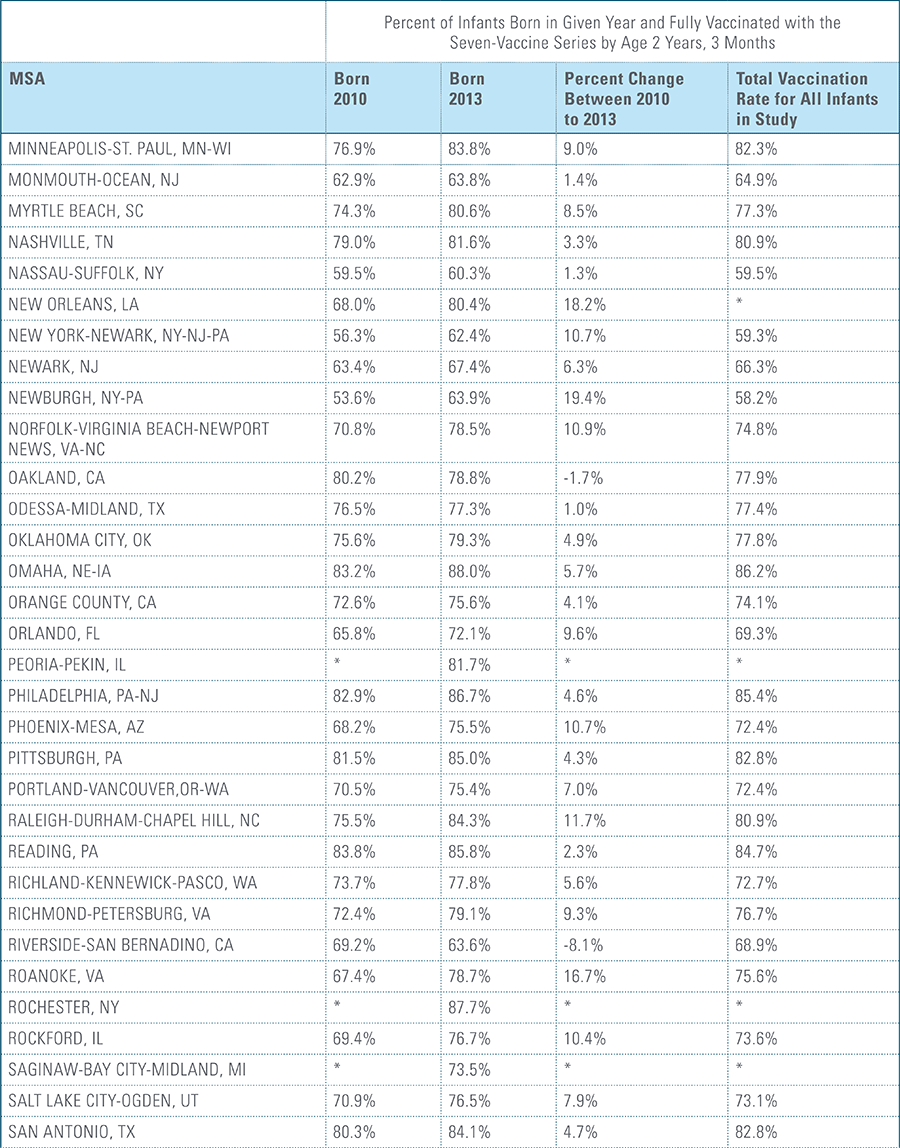
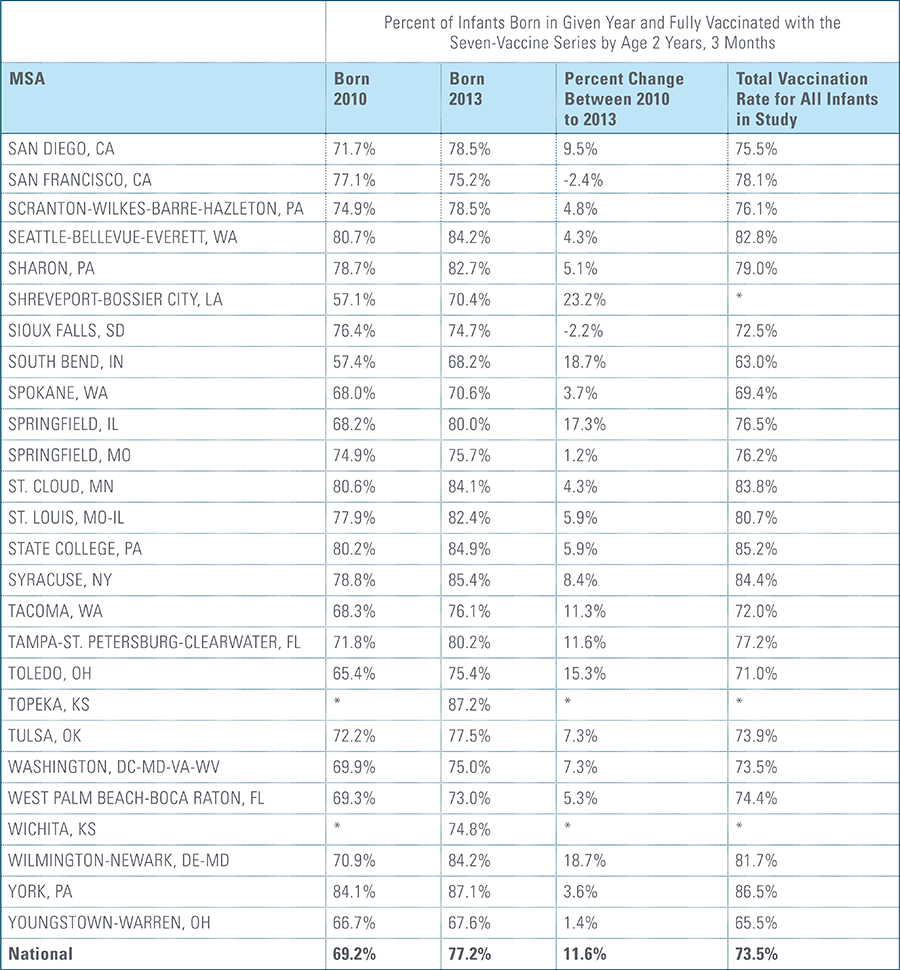
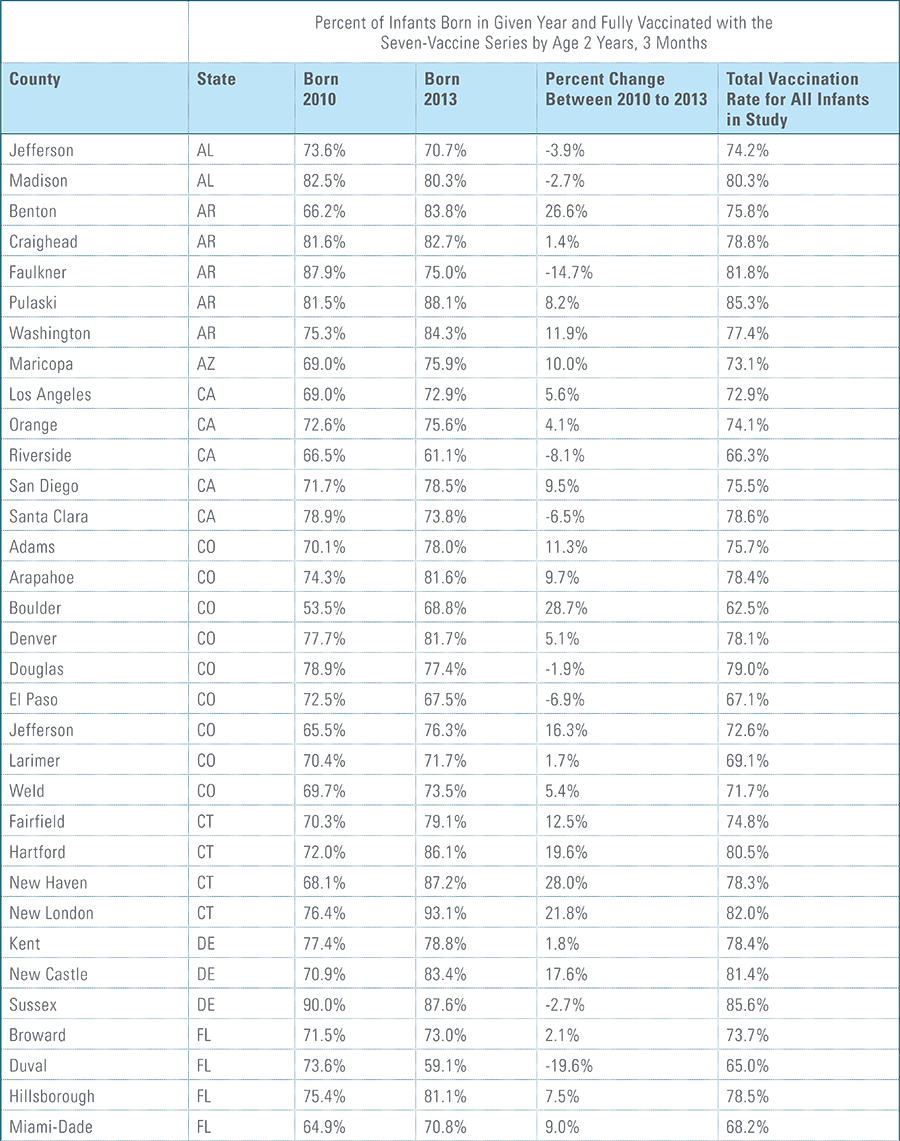
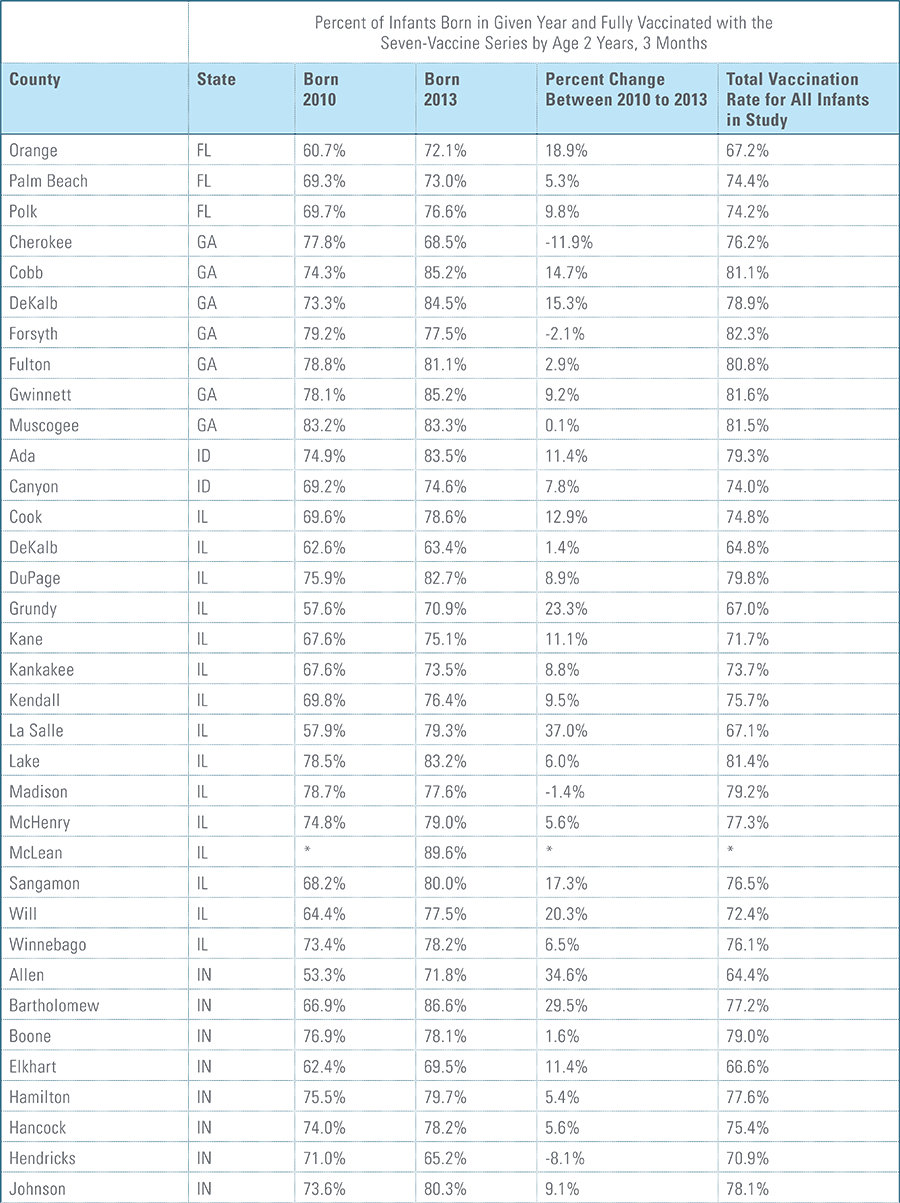
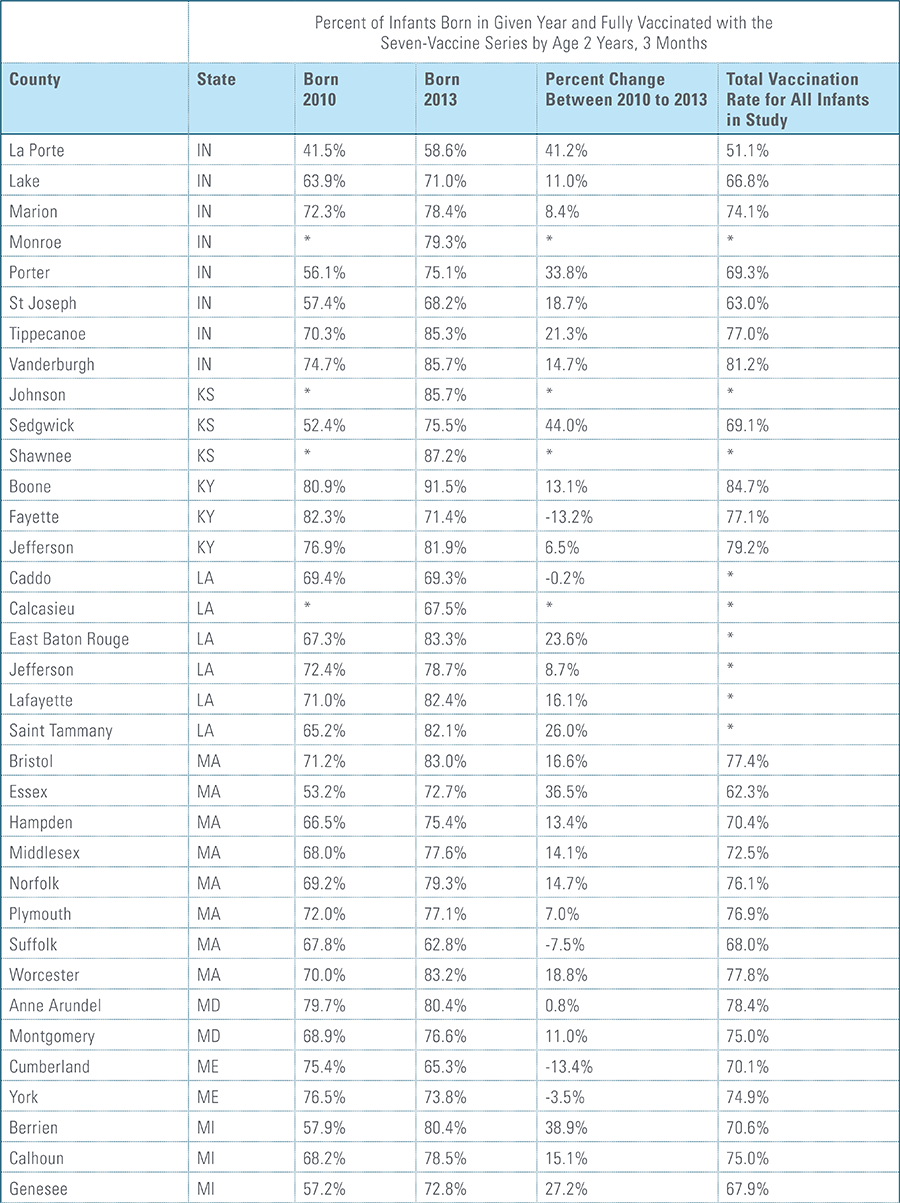
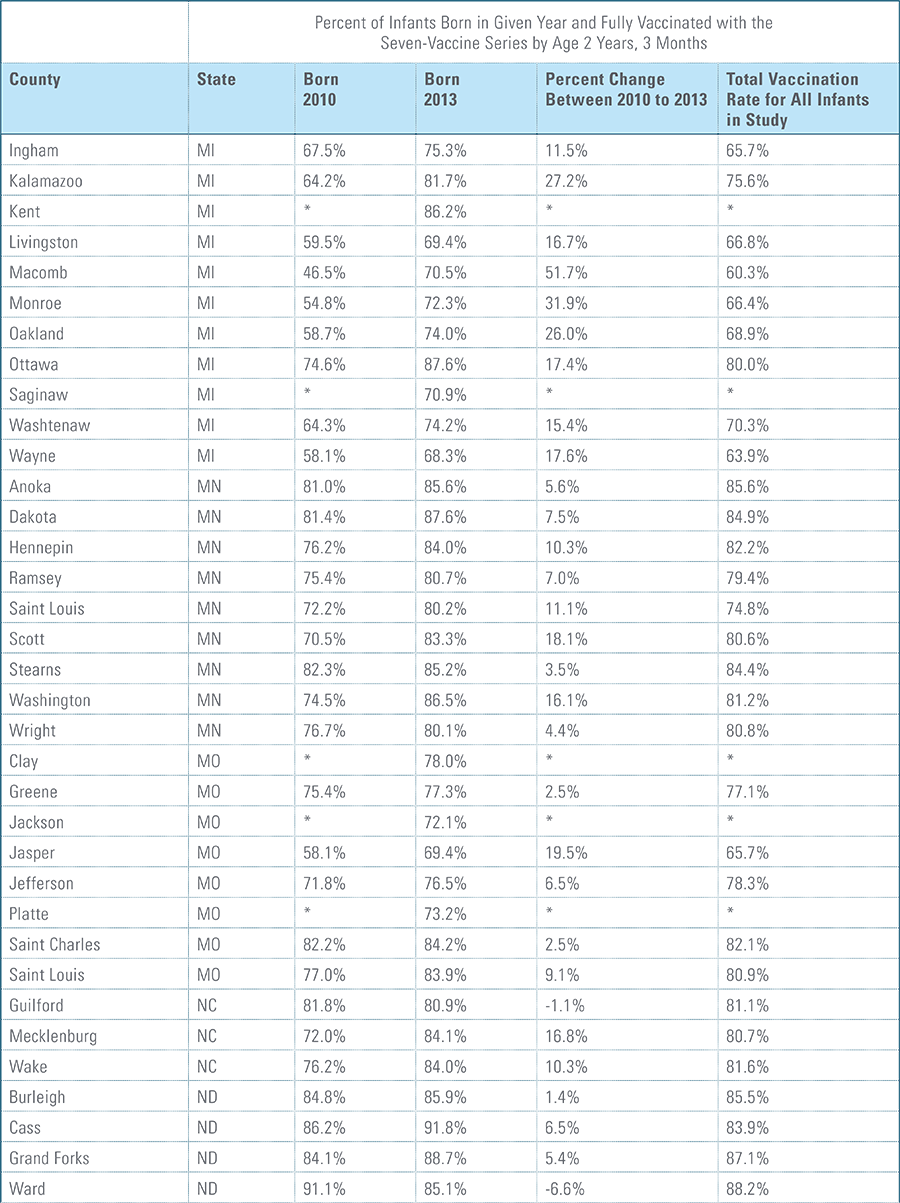
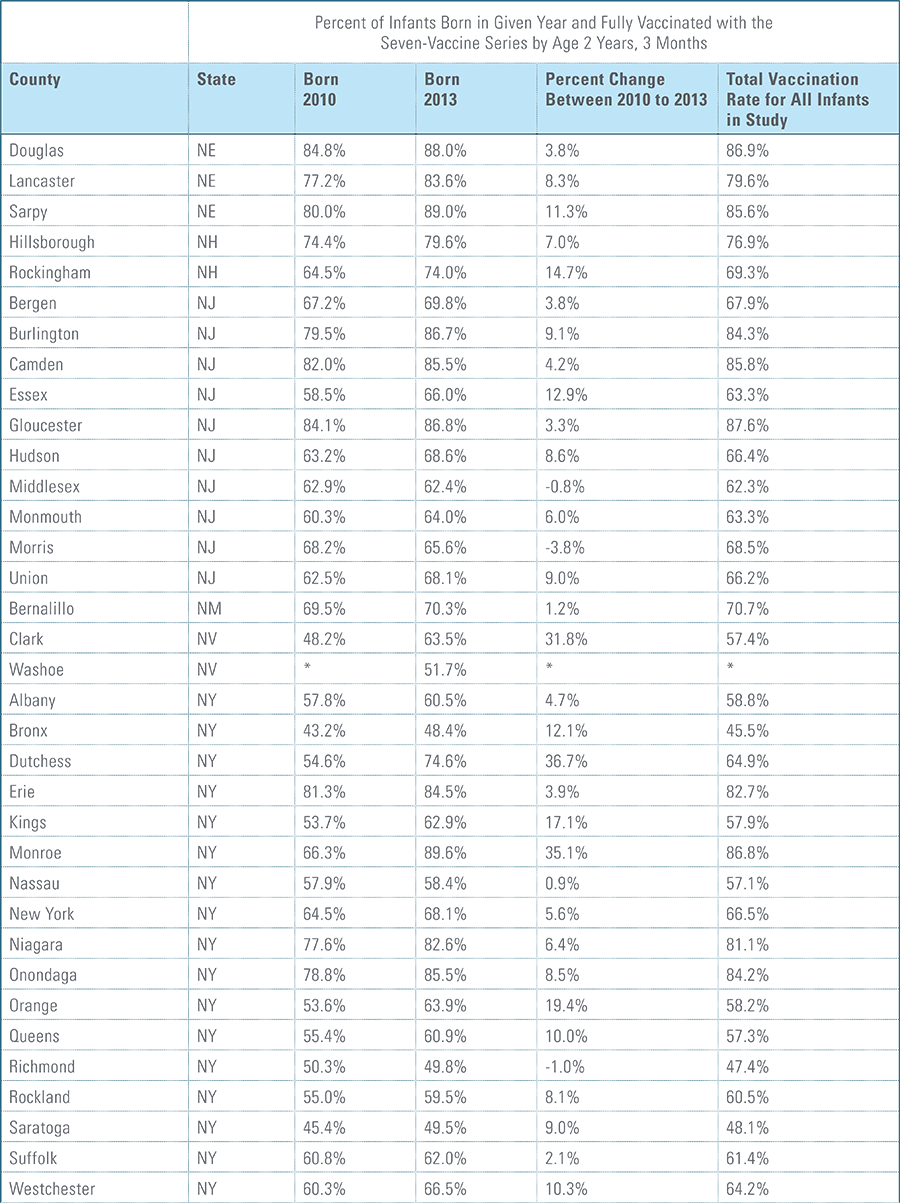
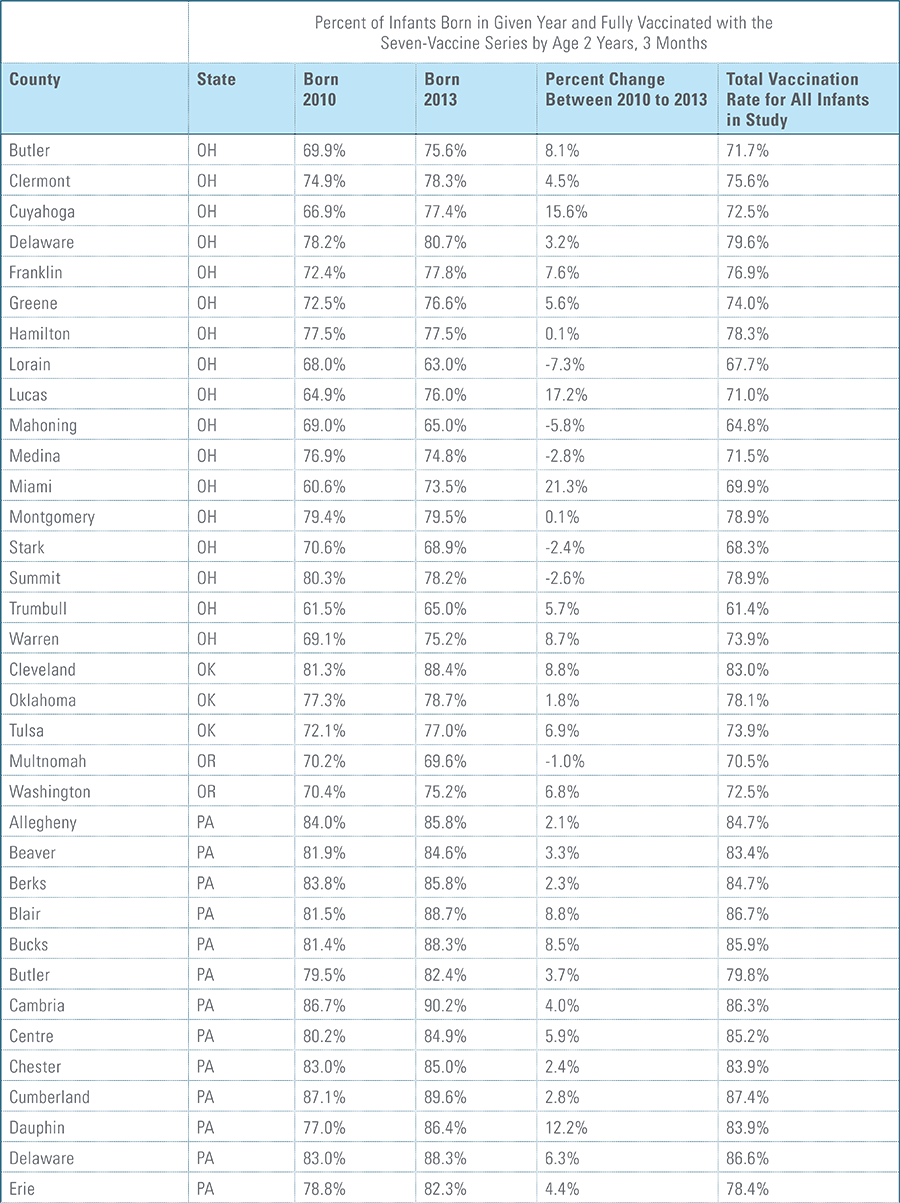
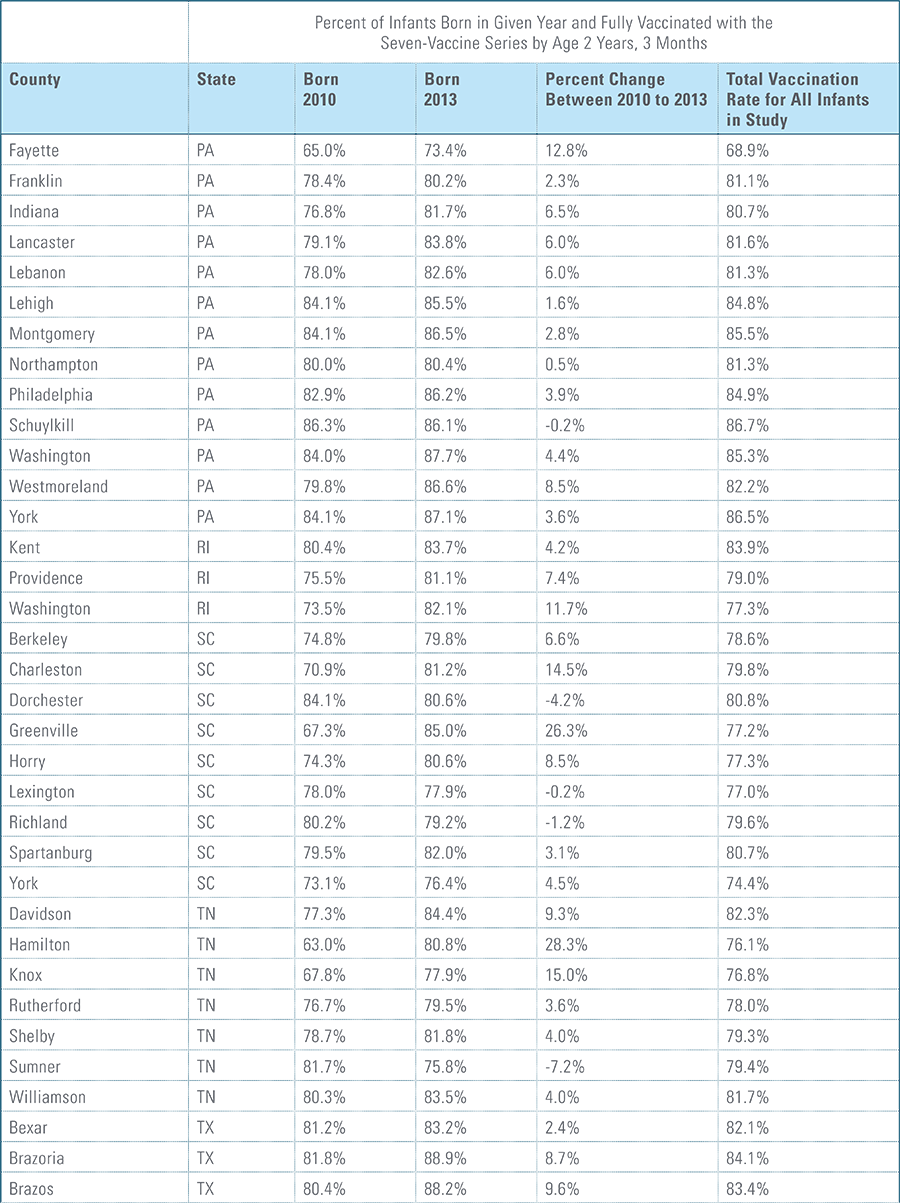
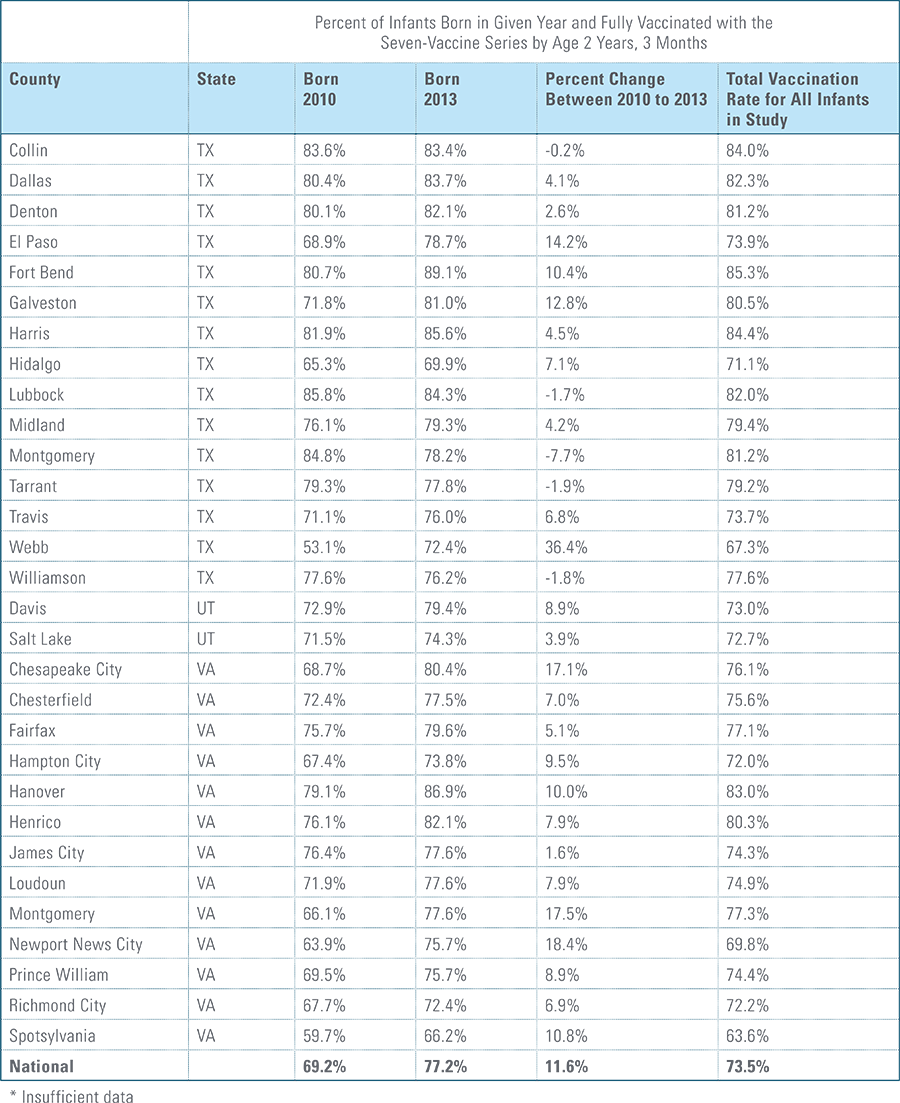
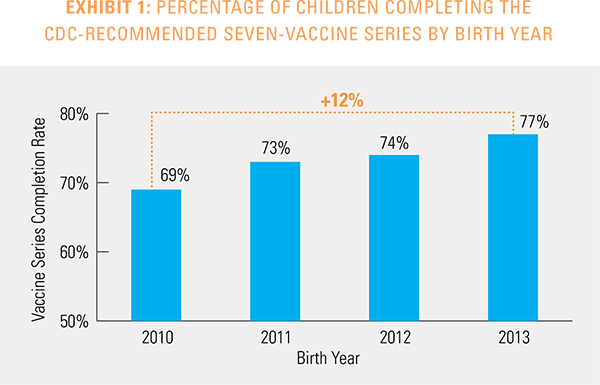 The proportion of children up-to-date on the CDC-recommended seven-vaccine series by 27 months of age rose steadily in recent years (see Exhibit 1). Completion rates have increased 12 percent nationally from 69 percent for children born in 2010 (who completed the series by 2013) to 77 percent for children born in 2013 (who completed the series by 2016).
The proportion of children up-to-date on the CDC-recommended seven-vaccine series by 27 months of age rose steadily in recent years (see Exhibit 1). Completion rates have increased 12 percent nationally from 69 percent for children born in 2010 (who completed the series by 2013) to 77 percent for children born in 2013 (who completed the series by 2016).